Cancer Res Treat.
2011 Dec;43(4):231-235.
Adjuvant Effect of IV Clodronate on the Delay of Bone Metastasis in High-Risk Prostate Cancer Patients: A Prospective Study
- Affiliations
-
- 1Department of Urology, Hospital 9 de Julho of Sao Paulo, Sao Paulo, Brazil. paulortrodrigues@uol.com.br
- 2Department of Urology, Hospital Beneficencia Portuguesa of Sao Paulo, Sao Paulo, Brazil.
Abstract
- PURPOSE
High-risk prostate cancer patients undergoing treatment often experience biochemical recurrence. The use of bisphosphonates as an adjuvant treatment delays skeletal events, yet whether or not bisphosphonates also delay metastastic development remains to be determined.
MATERIALS AND METHODS
A total of 140 high-risk prostate cancer patients who were undergoing definitive treatment and who had clinically organ-confined disease and who suffered from biochemical recurrence were administered intravenous (IV) clodronate. The patients were treated with a radical retropubic prostatectomy (RP) or curative radiotherapy (RTx). Upon androgen deprivation therapy initiation, tri-monthly IV clodronate was added to the treatment to prevent bone demineralization. Twenty-six out of 60 operated cases and 45 out of 80 irradiated cases received bisphosphonate. The length of time until the first bone metastasis was recorded and analyzed.
RESULTS
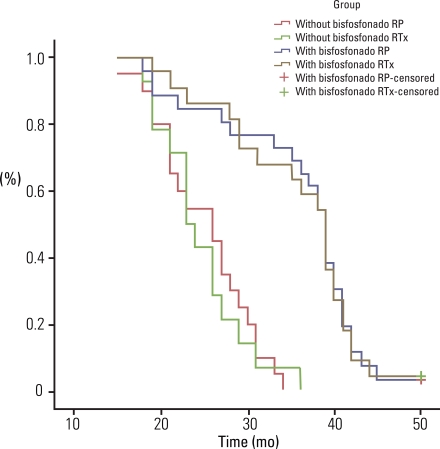
No statistical difference was found for the type of primary treatment (RP or RTx) on the time to the first bone metastasis (95% confidence interval [CI], 0.40 to 2.43; p=0.98). However, there was a clear advantage favoring the group that received bisphosphonate (p<0.001). The addition of bisphosphonate delayed the appearance of the first bone metastasis by seven-fold (95% CI, 3.1 to 15.4; p<0.001).
CONCLUSION
Treatment with tri-monthly IV clodronate delayed the time to the first bone metastasis in high-risk prostate cancer patients who were experiencing an increase in the prostate specific antigen level after definitive treatment.
Keyword
MeSH Terms
-
Androgen Antagonists
Clodronic Acid
Diphosphonates
Hormone Antagonists
Humans
Imidazoles
Neoplasm Metastasis
Nitro Compounds
Osteoporosis
Prospective Studies
Prostate
Prostate-Specific Antigen
Prostatectomy
Prostatic Neoplasms
Recurrence
Androgen Antagonists
Clodronic Acid
Diphosphonates
Hormone Antagonists
Imidazoles
Nitro Compounds
Prostate-Specific Antigen
Figure
Reference
-
1. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003; 169:517–523. PMID: 12544300.
Article2. Shipley WU, Thames HD, Sandler HM, Hanks GE, Zietman AL, Perez CA, et al. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999; 281:1598–1604. PMID: 10235152.
Article3. Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropinreleasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005; 103:1615–1624. PMID: 15742331.
Article4. Diel IJ, Solomayer EF, Bastert G. Bisphosphonates and the prevention of metastasis: first evidences from preclinical and clinical studies. Cancer. 2000; 88(12 Suppl):3080–3088. PMID: 10898355.5. Diel IJ. Antitumour effects of bisphosphonates: first evidence and possible mechanisms. Drugs. 2000; 59:391–399. PMID: 10776826.6. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974. PMID: 9749478.7. Whittemore DE, Hick EJ, Carter MR, Moul JW, Miranda-Sousa AJ, Sexton WJ. Significance of tertiary Gleason pattern 5 in Gleason score 7 radical prostatectomy specimens. J Urol. 2008; 179:516–522. PMID: 18076949.
Article8. Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-oflife considerations throughout the continuum of care. Eur Urol. 2004; 46:731–739. PMID: 15548440.
Article9. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002; 94:1458–1468. PMID: 12359855.
Article10. Rodrigues P, Hering F, Campagnari JC. Use of bisphosphonates can dramatically improve pain in advanced hormone-refractory prostate cancer patients. Prostate Cancer Prostatic Dis. 2004; 7:350–354. PMID: 15534620.
Article11. Rodrigues P, Hering FO, Bruna P, Meller A, Afonso Y. Comparative study of the protective effect of different intravenous bisphosphonates on the decrease in bone mineral density in patients submitted to radical prostatectomy undergoing androgen deprivation therapy: a prospective open-label controlled study. Int J Urol. 2007; 14:317–320. PMID: 17470161.
Article12. Ullen A, Lennartsson L, Hjelm-Eriksson M, Kälkner KM, Lennernäs B, Nilsoon S. Additive/synergistic anti-tumoral effects on prostate cancer cells in vitro following treatment with a combination of gencitabine and zoledronic acid. Proc Am Soc Clin Oncol. 2003; 22:1737.13. Daubiné F, Le Gall C, Gasser J, Green J, Clézardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007; 99:322–330. PMID: 17312309.14. Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002; 302:1055–1061. PMID: 12183663.
Article15. Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997; 57:3890–3894. PMID: 9307266.16. Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998; 339:357–363. PMID: 9691101.
Article17. Brawer MK. Radiation therapy failure in prostate cancer patients: risk factors and methods of detection. Rev Urol. 2002; 4(Suppl 2):S2–S11. PMID: 16986008.18. Saad F. Bisphosphonates can prevent skeletal complications of malignant bone disease from prostate cancer and renal cell carcinoma. Eur Urol Suppl. 2007; 6:683–688.
Article19. Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003; 169:2008–2012. PMID: 12771706.
Article20. Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007; 25:1038–1042. PMID: 17369566.
Article21. Mangiapane S, Hoer A, Gothe H, Barghout V, Haeussler B. Higher persistency with I.V. bisphosphonates in patients with bone metastasis. J Clin Oncol. 2006; 24(18S):18623.
Article22. Mason MD, Sydes MR, Glaholm J, Langley RE, Huddart RA, Sokal M, et al. Oral sodium clodronate for nonmetastatic prostate cancer: results of a randomized double-blind placebocontrolled trial: Medical Research Council PR04 (ISRCTN61384873). J Natl Cancer Inst. 2007; 99:765–776. PMID: 17505072.23. Powles T, McCroskey E, Paterson A. Oral bisphosphonates as adjuvant therapy for operable breast cancer. Clin Cancer Res. 2006; 12(20 Pt 2):6301s–6304s. PMID: 17062718.
Article24. Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006; 8:R13. PMID: 16542503.
Article25. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009; 10:872–876. PMID: 19674936.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Patients With High Risk Prostate Cancer
- The role of metastasis-directed therapy and local therapy of the primary tumor in the management of oligometastatic prostate cancer
- Change of Bone Mineral Density after 1,25(OH)2VitD Therapy In Newly Diagnosed Prostate Cancer Patients Treated with Total Androgen Blockade: The Prospective Study
- Solitary Testicular Metastasis of Prostate Cancer Mimicking Primary Testicular Cancer
- Choroidal Metastasis from Prostate Cancer