J Korean Med Sci.
2011 Dec;26(12):1634-1637. 10.3346/jkms.2011.26.12.1634.
Fatal Rhabdomyolysis in a Patient with Liver Cirrhosis after Switching from Simvastatin to Fluvastatin
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Inje University College of Medicine, Busan, Korea. thkim@paik.ac.kr
- KMID: 1786022
- DOI: http://doi.org/10.3346/jkms.2011.26.12.1634
Abstract
- HMG-CoA reductase inhibitors (statins) are widely used to treat hypercholesterolemia. Among the adverse effects associated with these drugs are statin-associated myopathies, ranging from asymptomatic elevation of serum creatine kinase to fatal rhabdomyolysis. Fluvastatin-induced fatal rhabdomyolysis has not been previously reported. We describe here a patient with liver cirrhosis who experienced fluvastatin-induced fatal rhabdomyolysis. This patient had been treated with simvastatin (20 mg/day) for coronary artery disease and was switched to fluvastatin (20 mg/day) 10 days before admission. He was also taking aspirin, betaxolol, candesartan, lactulose, and entecavir. Rhabdomyolysis was complicated and continued to progress. He was treated with massive hydration, urine alkalization, intravenous furosemide, and continuous renal replacement therapy for acute renal failure, but eventually died due to rhabdomyolysis complicated by hepatic failure. In conclusion, fluvastatin should be used with caution in patients with liver cirrhosis, especially with other medications metabolized with CYP2C9.
Keyword
MeSH Terms
-
Coronary Artery Disease/complications/*drug therapy
Fatal Outcome
Fatty Acids, Monounsaturated/administration & dosage/*adverse effects/therapeutic use
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors/administration & dosage/*adverse effects/therapeutic use
Indoles/administration & dosage/*adverse effects/therapeutic use
Liver Cirrhosis/*complications
Male
Middle Aged
Rhabdomyolysis/*chemically induced
Simvastatin/administration & dosage/therapeutic use
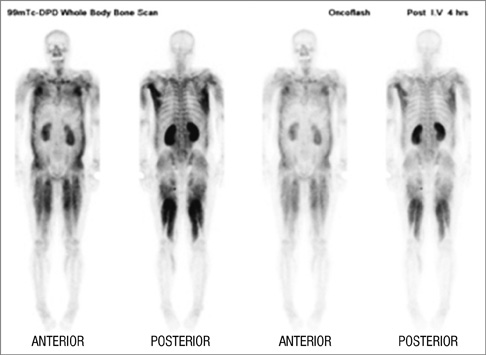
Figure
Reference
-
1. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.2. Suh JW, Choi DJ, Chang HJ, Cho YS, Youn TJ, Chae IH, Kim KI, Kim CH, Kim HS, Oh BH, Park YB. HMG-CoA reductase inhibitor improves endothelial dysfunction in spontaneous hypertensive rats via down-regulation of caveolin-1 and activation of endothelial nitric oxide synthase. J Korean Med Sci. 2010. 25:16–23.3. Corsini A, Jacobson TA, Ballantyne CM. Fluvastatin: clinical and safety profile. Drugs. 2004. 64:1305–1323.4. Liberopoulos EN, Daskalopoulou SS, Mikhailidis DP, Wierzbicki AS, Elisaf MS. A review of the lipid-related effects of fluvastatin. Curr Med Res Opin. 2005. 21:231–244.5. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002. 346:539–540.6. Akoglu H, Yilmaz R, Kirkpantur A, Arici M, Altun B, Turgan C. Combined organ failure with combination antihyperlipidemic treatment: a case of hepatic injury and acute renal failure. Ann Pharmacother. 2007. 41:143–147.7. Russo MW, Jacobson IM. How to use statins in patients with chronic liver disease. Cleve Clin J Med. 2004. 71:58–62.8. Cohen DE, Anania FA, Chalasani N. National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. Am J Cardiol. 2006. 97:77C–81C.9. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. American College of Medicine, Ameican Heart Association, National Heart Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002. 40:567–572.10. Zhang Q, Yang Z. Can statins be used safely in coronary heart disease patients of hepatitis B virus carriers? Int J Cardiol. 2011. 146:291.11. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002. 36:288–295.12. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004. 109:III50–III57.13. Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999. 84:413–428.14. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010. 340:c2197.15. Zateyshchikov DA, Minushkina LO, Brovkin AN, Savel'eva EG, Zateyshchikova AA, Manchaeva BB, Nikitin AG, Sidorenko BA, Nosikov VV. Association of CYP2D6 and ADRB1 genes with hypotensive and antichronotropic action of betaxolol in patients with arterial hypertension. Fundam Clin Pharmacol. 2007. 21:437–443.16. Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998. 45:525–538.17. Conforti A, Magro L, Moretti U, Scotto S, Motola D, Salvo F, Ros B, Leone R. Fluvastatin and hepatic reactions: a signal from spontaneous reporting in Italy. Drug Saf. 2006. 29:1163–1172.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rhabdomyolysis in a Cyclosporine-treated Renal Transplant Recipient Who Received Atorvastatin as Replacement for Fluvastatin
- Clinical Characteristics of Nontraumatic Rhabdomyolysis in Patients with Liver Cirrhosis
- Fenoverine-induced Rhabdomyolysis in a Patient with Liver Cirrhosis
- Two Cases of Rhabdomyolysis Developed in Patients with Liver Cirrhosis
- Two Cases of Simvastatin-induced Acute Myopathy