J Korean Med Sci.
2011 Dec;26(12):1576-1581. 10.3346/jkms.2011.26.12.1576.
Role of the Alternans of Action Potential Duration and Aconitine-Induced Arrhythmias in Isolated Rabbit Hearts
- Affiliations
-
- 1Department of Cardiology, Fatima General Hospital, Daegu, Korea.
- 2Cardiovascular Division, Internal Medicine, Aging-Associated Vascular Disease Research Center, Yeungnam University Medical Center, Daegu, Korea. dgshin@med.yu.ac.kr
- 3Cardiovascular Department, Kyungpook National University, Daegu, Korea.
- 4Cardiovascular Division, Internal Medicine, Keimyumg University, Daegu, Korea.
- 5Cardiovascular Division, Department of Internal Medicine, Daegu Catholic University, Daegu, Korea.
- KMID: 1786013
- DOI: http://doi.org/10.3346/jkms.2011.26.12.1576
Abstract
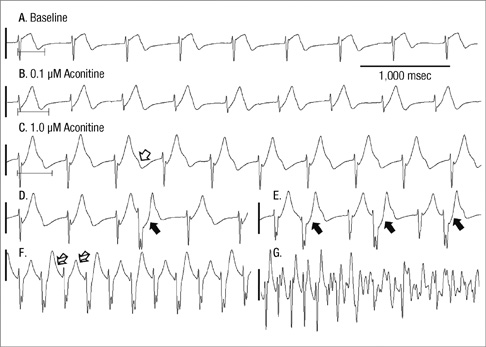
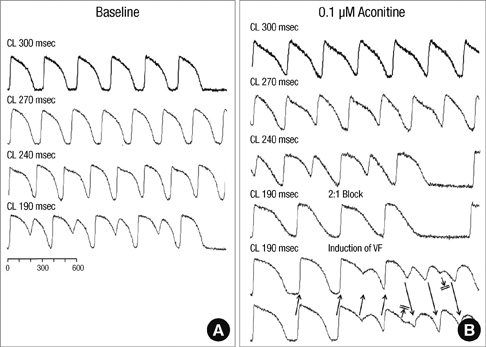
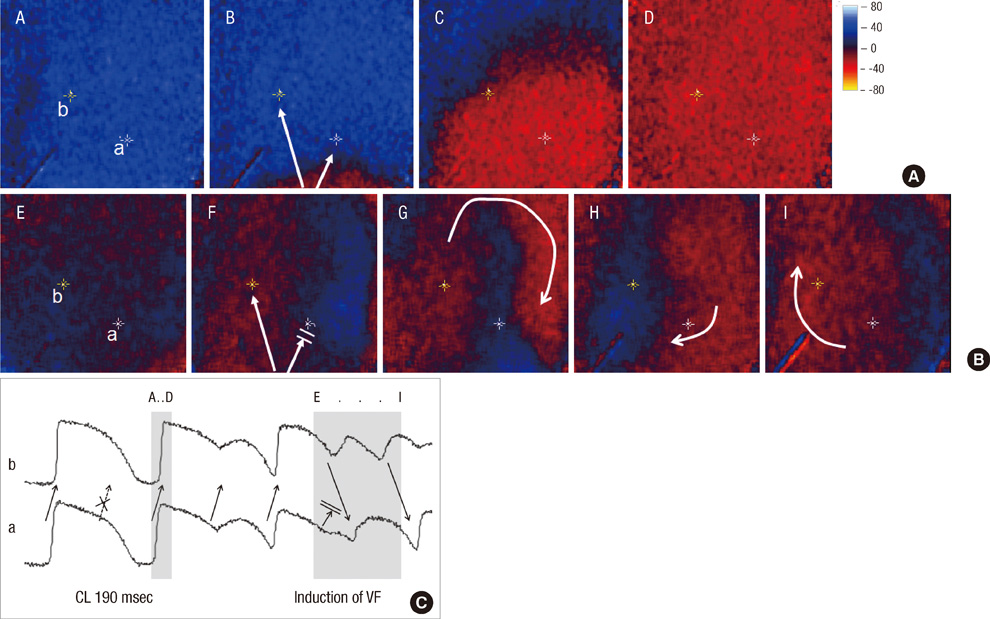
- Under conditions of Na+ channel hyperactivation with aconitine, the changes in action potential duration (APD) and the restitution characteristics have not been well defined in the context of aconitine-induced arrhythmogenesis. Optical mapping of voltage using RH237 was performed with eight extracted rabbit hearts that were perfused using the Langendorff system. The characteristics of APD restitution were assessed using the steady-state pacing protocol at baseline and 0.1 microM aconitine concentration. In addition, pseudo-ECG was analyzed at baseline, and with 0.1 and 1.0 microM of aconitine infusion respectively. Triggered activity was not shown in dose of 0.1 microM aconitine but overtly presented in 1.0 microM of aconitine. The slopes of the dynamic APD restitution curves were significantly steeper with 0.1 microM of aconitine than at baseline. With aconitine administration, the cycle length of initiation of APD alternans was significantly longer than at baseline (287.5 +/- 9.6 vs 247.5 +/- 15.0 msec, P = 0.016). The functional reentry following regional conduction block appears with the progression of APD alternans. Ventricular fibrillation is induced reproducibly at pacing cycle length showing a 2:1 conduction block. Low-dose aconitine produces arrhythmogenesis at an increasing restitution slope with APD alternans as well as regional conduction block that proceeds to functional reentry.
Keyword
MeSH Terms
-
Aconitine/*pharmacology
Action Potentials/*drug effects
Animals
Arrhythmias, Cardiac/*chemically induced/*physiopathology
Cardiac Pacing, Artificial
Electrocardiography
Heart/physiopathology
Heart Conduction System/physiology
Myocardium/*pathology
Rabbits
Sodium Channels/drug effects/metabolism
Ventricular Fibrillation/physiopathology
Figure
Reference
-
1. Friese J, Gleitz J, Gutser UT, Heubach JF, Matthiesen T, Wilffert B, Selve N. Aconitum sp. alkaloids: the modulation of voltage-dependent Na+ channels, toxicity and antinociceptive properties. Eur J Pharmacol. 1997. 337:165–174.2. Kimura I, Makino M, Takamura Y, Islam MA, Kimura M. Positive chronotropic and inotropic effects of higenamine and its enhancing action on the aconitine-induced tachyarrhythmia in isolated murine atria. Jpn J Pharmacol. 1994. 66:75–80.3. Catterall WA. Structure and function of voltage-sensitive ion channels. Science. 1988. 242:50–61.4. Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol. 2000. 36:1–12.5. Koller ML, Riccio ML, Gilmour RF Jr. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998. 275:H1635–H1642.6. Banville I, Chattipakorn N, Gray RA. Restitution dynamics during pacing and arrhythmias in isolated pig hearts. J Cardiovasc Electrophysiol. 2004. 15:455–463.7. Wright SN. Comparison of aconitine-modified human heart (hH1) and rat skeletal (µ1) muscle Na+ channels: an important role for external Na+ ions. J Physiol. 2002. 538:759–771.8. Chan TY. Aconite poisoning. Clin Toxicol. 2009. 47:279–285.9. Arita J, Xue XY, Aye NN, Fukuyama K, Wakui Y, Niitsu K, Maruno M, Siying C, Hashimoto K. Antiarrhythmic effects of an aconitine-like compound, TJN-505, on canine arrhythmia models. Eur J Pharmacol. 1996. 318:333–340.10. Nattel S, Khairy P, Schram G. Arrhythmogenic ionic remodeling: adaptive responses with maladaptive consequences. Trends Cardiovasc Med. 2001. 11:295–301.11. Li Y, Tu D, Xiao H, Du Y, Zou A, Liao Y, Dong S. Aconitine blocks HERG and Kv1.5 potassium channels. J Ethnopharmacol. 2010. 131:187–195.12. Wang YJ, Chen BS, Lin MW, Lin AA, Peng H, Sung RJ, Wu SN. Time-dependent block of ultrarapid-delayed rectifier K+ currents by aconitine, a potent cardiotoxin, in heart-derived H9c2 myoblasts and in neonatal rat ventricular myocytes. Toxicol Sci. 2008. 106:454–463.13. Cranefield PF. Action potentials, afterpotentials, and arrhythmias. Circ Res. 1977. 41:415–423.14. Adaniya H, Hayami H, Hiraoka M, Sawanobori T. Effects of magnesium on polymorphic ventricular tachycardias induced by aconitine. J Cardiovasc Pharmacol. 1994. 24:721–729.15. Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol. 1968. 25:191–196.16. Elharrar V, Surawicz B. Cycle length effect on restitution of action potential duration in dog cardiac fibers. Am J Physiol. 1983. 244:H782–H792.17. Garfinkel A, Chen PS, Walter DO, Karagueuzian HS, Kogan B, Evans SJ, Karpoukhin M, Hwang C, Uchida T, Gotoh M, Nwasokwa O, Sager P, Weiss JN. Quasiperiodicity and chaos in cardiac fibrillation. J Clin Invest. 1997. 99:305–314.18. Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000. 102:1664–1670.19. Wilson LD, Rosenbaum DS. Mechanisms of arrhythmogenic cardiac alternans. Europace. 2007. 9:vi77–vi82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Changes of the Visual Evoked Potential for Aconitine Induced Visual Pathway Demyelination in Rabbit Model
- Case of Ventricular Tachycardia After Caowu-Ingestion
- Experimental Aconitine Neuroretinopathy in Rabbit Eye
- Cardiovascular aspects of aconitine poisoning
- Rabltit Model of Acenitine Myelo-optic Neuropathy