J Korean Med Sci.
2011 Oct;26(10):1371-1377. 10.3346/jkms.2011.26.10.1371.
Pharmacology of Intracisternal or Intrathecal Glycine, Muscimol, and Baclofen in Strychnine-induced Thermal Hyperalgesia of Mice
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, College of Medicine, Korea University, Seoul, Korea. iloklee@korea.ac.kr
- 2Department of Anesthesiology, Madi Hospital, Seoul, Korea.
- 3Department of Plastic Surgery, School of Medicine, Inha University, Incheon, Korea.
- KMID: 1785997
- DOI: http://doi.org/10.3346/jkms.2011.26.10.1371
Abstract
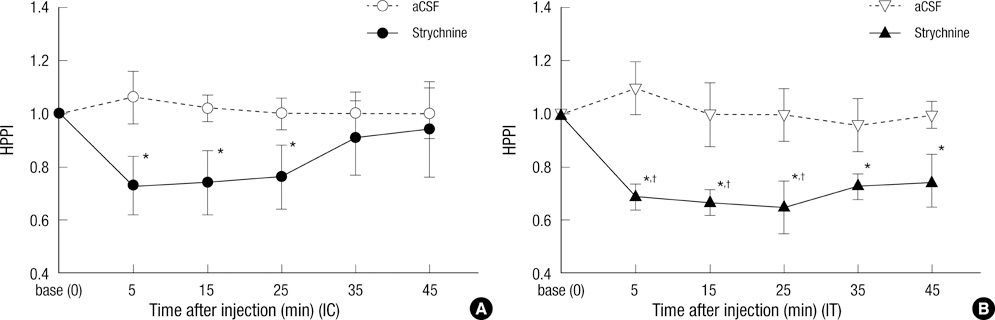
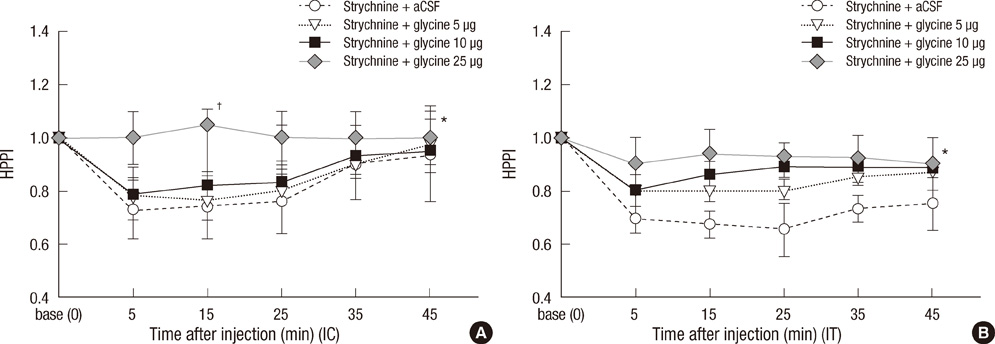
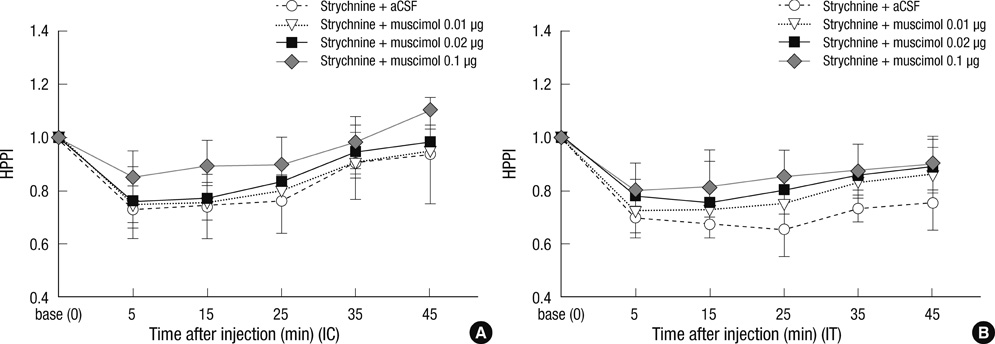
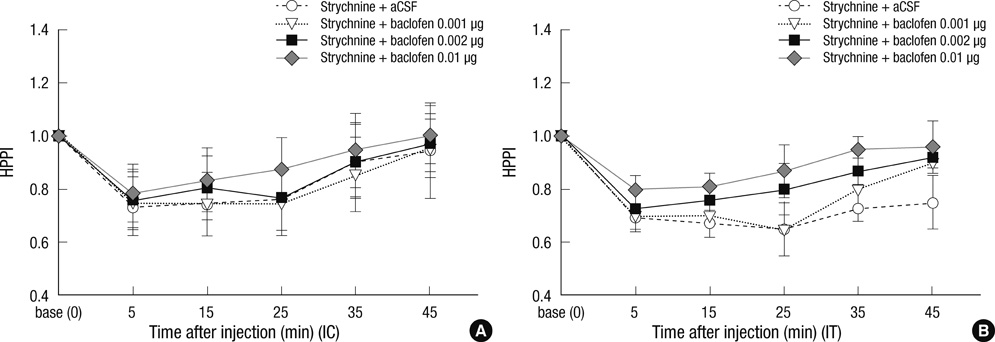
- Glycine and gamma-aminobutyric acid (GABA) are localized and released by the same interneurons in the spinal cord. Although the effects of glycine and GABA on analgesia are well known, little is known about the effect of GABA in strychnine-induced hyperalgesia. To investigate the effect of GABA and the role of the glycine receptor in thermal hyperalgesia, we designed an experiment involving the injection of muscimol (a GABAA receptor agonist), baclofen (a GABAB receptor agonist) or glycine with strychnine (strychnine sensitive glycine receptor antagonist). Glycine, muscimol, or baclofen with strychnine was injected into the cisterna magna or lumbar subarachnoidal spaces of mice. The effects of treatment on strychnine-induced heat hyperalgesia were observed using the pain threshold index via the hot plate test. The dosages of experimental drugs and strychnine we chose had no effects on motor behavior in conscious mice. Intracisternal or intrathecal administration of strychnine produced thermal hyperalgesia in mice. Glycine antagonize the effects of strychnine, whereas, muscimol or baclofen does not. Our results indicate that glycine has anti-thermal hyperalgesic properties in vivo; and GABA receptor agonists may lack the binding abilities of glycine receptor antagonists with their sites in the central nervous system.
Keyword
MeSH Terms
-
Animals
Baclofen/*administration & dosage/pharmacology
Drug Delivery Systems
GABA Agonists/administration & dosage/pharmacology
GABA Antagonists/administration & dosage/pharmacology
Glycine/*administration & dosage/pharmacology
Hot Temperature
Hyperalgesia/chemically induced/*drug therapy
Injections, Spinal
Male
Mice
Mice, Inbred ICR
Muscimol/*administration & dosage/pharmacology
Pain Threshold
Random Allocation
Strychnine
gamma-Aminobutyric Acid/metabolism
Figure
Reference
-
1. Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960. 6:117–141.2. Mitchell K, Spike RC, Todd AJ. An immunocytochemical study of glycine receptor and GABA in laminae I-III of rat spinal dorsal horn. J Neurosci. 1993. 13:2371–2381.3. Jonas P, Bischofberger J, Sandkühler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998. 281:419–424.4. Chen Y, Dai TJ, Zeng YM. Strychnine-sensitive glycine receptors mediate the analgesic but not hypnotic effects of emulsified volatile anesthetics. Pharmacology. 2007. 80:151–157.5. Lynch JW, Callister RJ. Glycine receptors: a new therapeutic target in pain pathways. Curr Opin Investig Drugs. 2006. 7:48–53.6. Lee IO, Lim ES. Intracisternal or intrathecal glycine, taurine, or muscimol inhibit bicuculline-induced allodynia and thermal hyperalgesia in mice. Acta Pharmacol Sin. 2010. 31:907–914.7. Lim ES, Lee IO. Effect of intrathecal glycine and related amino acids on the allodynia and hyperalgesic action of strychnine or bicuculline in mice. Korean J Anesthesiol. 2010. 58:76–86.8. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980. 67:313–316.9. Jobe PC, Dailey JW. Neurobiology of seizure predisposition in the genetically epilepsy prone rat. Proc West Pharmacol Soc. 1991. 34:223–225.10. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004. 111:116–124.11. Ewen A, Archer DP, Samanani N, Roth SH. Hyperalgesia during sedation: effects of barbiturates and propofol in the rat. Can J Anaesth. 1995. 42:532–540.12. Minami T, Uda R, Horiguchi S, Ito S, Hyodo M, Hayaishi O. Allodynia evoked by intrathecal administration of prostaglandin E2 to conscious mice. Pain. 1994. 57:217–223.13. Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989. 37:111–123.14. Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988. 32:89–94.15. Shir Y, Seltzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci Lett. 1990. 115:62–67.16. Yamamoto T, Yaksh TL. Effects of intrathecal strychnine and bicuculline on nerve compression-induced thermal hyperalgesia and selective antagonism by MK-801. Pain. 1993. 54:79–84.17. Cheng W, Yin Q, Cheng MY, Chen HS, Wang S, Feng T, Zeng YM, Liu GJ. Intracerebroventricular or intrathecal injection of glycine produces analgesia in thermal nociception and chemical nociception via glycine receptors. Eur J Pharmacol. 2009. 614:44–49.18. Hammond DL, Drower EJ. Effects of intrathecally administered THIP, baclofen and muscimol on nociceptive threshold. Eur J Pharmacol. 1984. 103:121–125.19. Wilson PR, Yaksh TL. Baclofen is antinociceptive in the spinal intrathecal space of animals. Eur J Pharmacol. 1978. 51:323–330.20. Aanonsen LM, Wilcox GL. Muscimol, gamma-aminobutyric acidA receptors and excitatory amino acids in the mouse spinal cord. J Pharmacol Exp Ther. 1989. 248:1034–1038.21. Hao JX, Xu IS, Xu XJ, Wiesenfeld-Hallin Z. Effects of intrathecal morphine, clonidine and baclofen on allodynia after partial sciatic nerve injury in the rat. Acta Anaesthesiol Scand. 1999. 43:1027–1034.22. Balerio GN, Rubio MC. Baclofen analgesia: involvement of the GABAergic system. Pharmacol Res. 2002. 46:281–286.23. Chung KM, Kim YH, Song DK, Huh SO, Suh HW. Differential modulation by baclofen on antinociception induced by morphine and beta-endorphin administered intracerebroventricularly in the formalin test. Neuropeptides. 1999. 33:534–541.24. Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002. 96:1161–1167.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of intrathecal glycine and related amino acids on the allodynia and hyperalgesic action of strychnine or bicuculline in mice
- Differential Role of Central GABA Receptors in Nociception of Orofacial Area in Rats
- The Effect of Intrathecal ACEA 1021, Competitive Glycine-site NMDA Receptor Antagonist on the Hyperalgesia Observed after Thermal Injury in the Rat
- Anxiolytic Action of Taurine via Intranasal Administration in Mice
- The effect of intrathecal baclofen single injection on neuropathic pain