J Korean Med Sci.
2010 Sep;25(9):1296-1304. 10.3346/jkms.2010.25.9.1296.
Aldosterone Modulates Cell Proliferation and Apoptosis in the Neonatal Rat Heart
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine, Korea University Hospital, Seoul, Korea. JGYNHG@yahoo.co.kr
- KMID: 1785908
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1296
Abstract
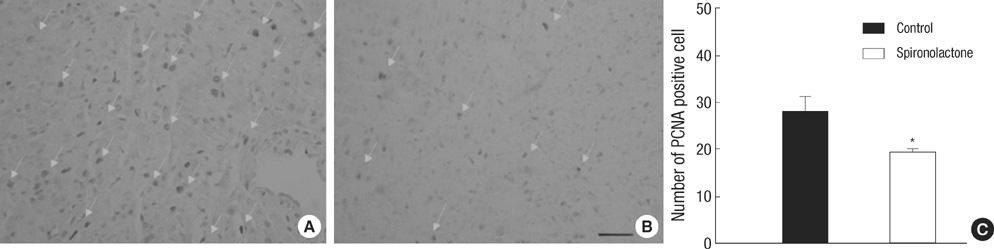
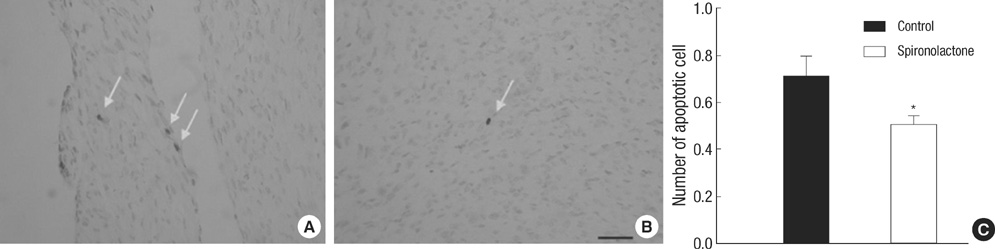
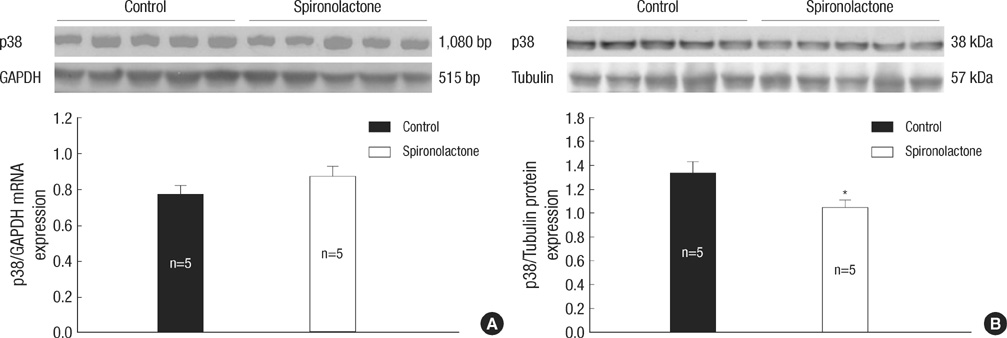
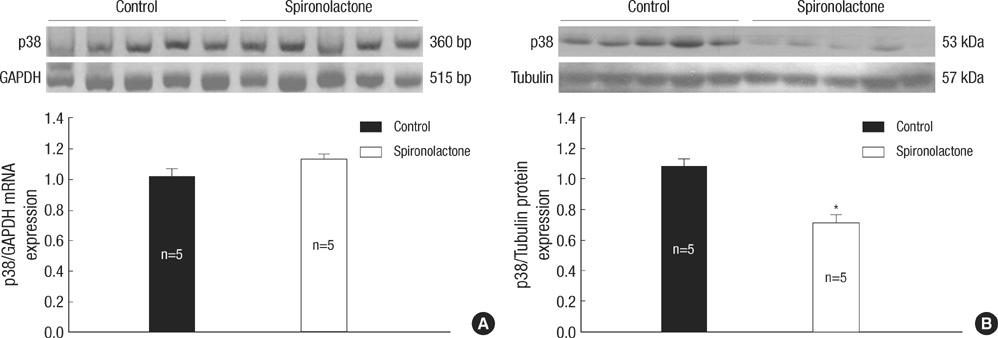
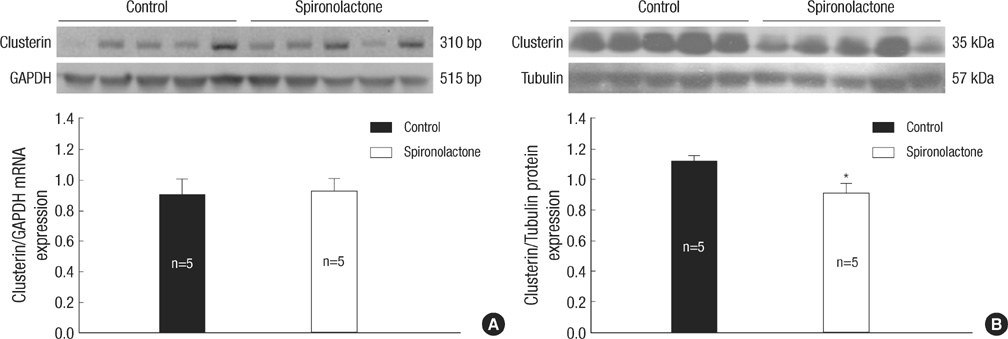
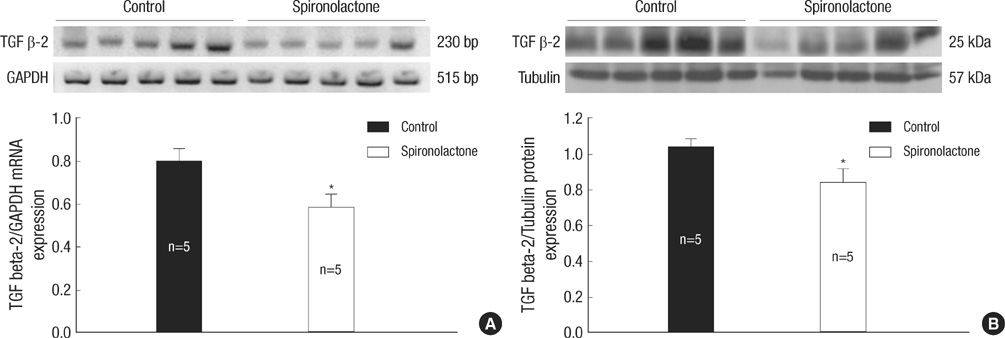
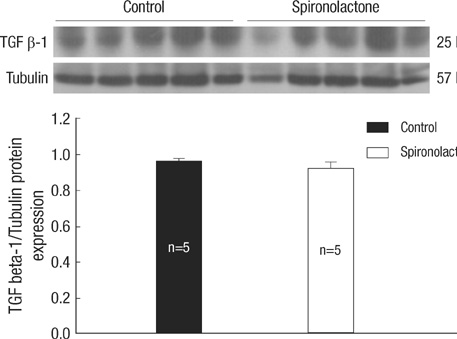
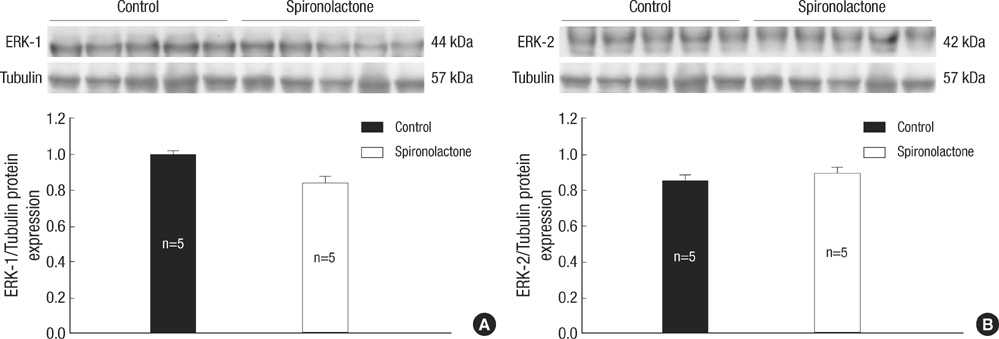
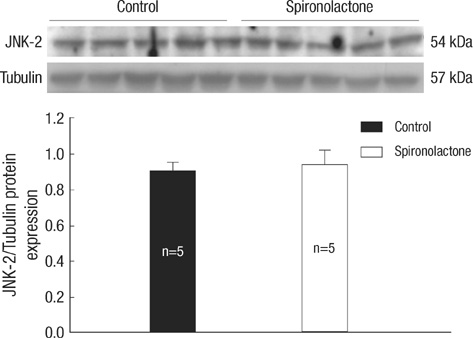
- In the present study, we investigated whether and how the mineralocorticoid receptor antagonist spironolactone affects cardiac growth and development through apoptosis and cell proliferation in the neonatal rat heart. Newborn rat pups were treated with spironolactone (200 mg/kg/d) for 7 days. The cell proliferation was studied by PCNA immunostaining. The treatment with spironolactone decreased proliferating myocytes by 32% (P<0.05), and reduced myocytes apoptosis by 29% (P<0.05). Immunoblot and immunohistochemistry for the expression of p38, p53, clusterin, TGF-beta2, and extracellular signal-regulated kinase were performed. In the spironolactone group, p38, p53, clusterin, and TGF-beta2 protein expression was significantly decreased (P<0.05). These results indicate that aldosterone inhibition in the developing rat heart induces cardiac growth impairment by decreasing proliferation and apoptosis of myocytes.
Keyword
MeSH Terms
-
Aldosterone Antagonists/*pharmacology
Animals
Animals, Newborn
*Apoptosis
Cell Proliferation
Clusterin/genetics/metabolism
Female
Heart/*drug effects/growth & development
Proliferating Cell Nuclear Antigen/metabolism
Rats
Rats, Sprague-Dawley
Spironolactone/*pharmacology
Transforming Growth Factor beta2/genetics/metabolism
Tumor Suppressor Protein p53/genetics/metabolism
p38 Mitogen-Activated Protein Kinases/genetics/metabolism
Figure
Reference
-
1. Choi JH, Yoo KH, Cheon HW, Kim KB, Hong YS, Lee JW, Kim SK, Kim CH. Angiotensin converting enzyme inhibition decreases cell turnover in the neonatal rat heart. Pediatr Res. 2002. 52:325–332.
Article2. Burniston JG, Saini A, Tan LB, Goldspink DF. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J Mol Cell Cardiol. 2005. 39:395–399.
Article3. Veliotes DG, Woodiwiss A, Deftereos DA, Gray D, Osadchii O, Norton GR. Aldosterone receptor blockade prevents the transition to cardiac pump dysfunction induced by beta-adrenoreceptor activation. Hypertension. 2005. 45:914–920.4. Wang W, McClain JM, Zucker IH. Aldosterone reduces baroreceptor discharge in the dog. Hypertension. 1992. 19:270–277.
Article5. Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005. 186:1–20.
Article6. Rocha R, Stier CT Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000. 141:3871–3878.
Article7. Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998. 82:1111–1129.8. Mano A, Tatsumi T, Shiraishi J, Keira N, Nomura T, Takeda M, Nishikawa S, Yamanaka S, Matoba S, Kobara M, Tanaka H, Shirayama T, Takamatsu T, Nozawa Y, Matsubara H. Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation. 2004. 110:317–323.
Article9. Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995. 270:1326–1331.
Article10. Leri A, Fiordaliso F, Setoguchi M, Limana F, Bishopric NH, Kajstura J, Webster K, Anversa P. Inhibition of p53 function prevents renin-angiotensin system activation and stretch-mediated myocyte apoptosis. Am J Pathol. 2000. 157:843–857.
Article11. Moses HL, Yang EY, Pietenpol JA. TGF-stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990. 63:245–247.12. Gallego M, Espina L, Vegas L, Echevarria E, Iriarte MM, Casis O. Spironolactone and captopril attenuates isoproterenol-induced cardiac remodelling in rats. Pharmacol Res. 2001. 44:311–315.
Article13. Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure. Circ Res. 2001. 88:298–304.
Article14. De Angelis N, Fiordaliso F, Latini R, Calvillo L, Funicello M, Gobbi M, Mennini T, Masson S. Appraisal of the role of angiotensin II and aldosterone in ventricular myocyte apoptosis in adult normotensive rat. J Mol Cell Cardiol. 2002. 34:1655–1665.
Article15. Beinlich CJ, White GJ, Baker KM, Morgan HE. Angiotensin II and left ventricular growth in newborn pig heart. J Mol Cell Cardiol. 1991. 23:1031–1038.
Article16. Struthers AD. Aldosterone-induced vasculopathy. Mol Cell Endocrinol. 2004. 217:239–241.
Article17. Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992. 20:248–254.
Article18. Engelmann GL, Vitullo JC, Gerrity RG. Morphometric analysis of cardiac hypertrophy during development, maturation, and senescence in spontaneously hypertensive rats. Circ Res. 1987. 60:487–494.
Article19. Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001. 98:12283–12288.
Article20. Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997. 29:859–870.21. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995. 80:293–299.
Article22. Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992. 70:523–526.
Article23. Pierzchalski P, Reiss K, Cheng W, Cirielli C, Kajstura J, Nitahara JA, Rizk M, Capogrossi MC, Anversa P. p53 induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res. 1997. 234:57–65.
Article24. Hochgrebe TT, Humphreys D, Wilson MR, Easterbrook-Smith SB. A reexamination of the role of clusterin as a complement regulator. Exp Cell Res. 1999. 249:13–21.
Article25. Zhou W, Janulis L, Park II, Lee C. Novel anti-proliferative property of clusterin in prostate cancer cells. Life Sci. 2002. 72:11–21.26. Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGF beta2 and TGF beta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999. 208:530–545.27. Singla DK, Sun B. Transforming growth factor-beta2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun. 2005. 332:135–141.28. Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001. 103:2745–2752.29. Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000. 19:6341–6350.
Article30. Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003. 35:1385–1394.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Endothelin A Receptor Antagonist to Cellular Proliferation and Apoptosis in Neonatal Rat Heart
- The Effect of Tumor Necrosis Factor-alpha in Cultured Neonatal Rat Cardiomyocytes
- A Study on the Change of Plasma Renin Activity(PRA) and Aldosterone Concentration(PAC) before and after Heart Operation in Children with Congenital Heart Disease
- Renal-specific Regulation of Apoptosis and It's Modulators by Angiotensin II
- Effect of Furosemide on the Morphogenesis of Neonatal Rat Renal Papilla