J Korean Med Sci.
2010 Sep;25(9):1291-1295. 10.3346/jkms.2010.25.9.1291.
Prostate Specific Membrane Antigen mRNA in Blood as a Potential Predictor of Biochemical Recurrence after Radical Prostatectomy
- Affiliations
-
- 1Center for Prostate Cancer, Goyang, Korea. uroonco@ncc.re.kr
- 2Department of Pathology, National Cancer Center, Goyang, Korea.
- KMID: 1785907
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1291
Abstract
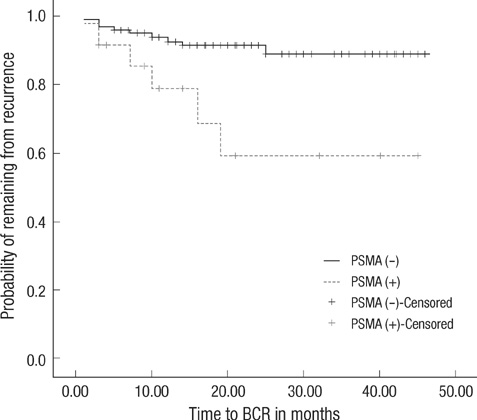
- We investigated whether the detection of prostate specific membrane antigen (PSMA) in blood preoperatively has predictive value for biochemical recurrence (BCR) after radical prostatectomy in patients with prostate cancer. All 134 patients scheduled to receive radical prostatectomy for prostate cancer were prospectively enrolled. The authors used nested reverse transcriptase-polymerase chain reaction (RT-PCR) assay to detect PSMA mRNA-bearing cells in peripheral blood, and analyzed the ability of PSMA mRNA positivity to predict BCR after surgery. PSMA-mRNA was detected in 24 (17.9%) patients by RT-PCR. Over a median follow-up of 20 months (range, 3 to 46 months), BCR developed in 15 patients (11.2%) and median time to BCR was 7 months (range, 3 to 25 months). Kaplan-Meier analysis revealed a significant difference between those positive or negative for PSMA in terms of recurrence-free actuarial probability (log rank P=0.0039). Multivariate analysis showed that positivity for PSMA mRNA (HR: 3.697, 95% CI 1.285-10.634, P=0.015) and a biopsy Gleason score of > or =7 (HR: 4.500, 95% CI 1.419-14.274, P=0.011) were independent preoperative predictors of BCR. The presence of PSMA mRNA in peripheral blood can be used to predict BCR after radical prostatectomy.
MeSH Terms
-
Aged
Antigens, Surface/*blood/genetics
Glutamate Carboxypeptidase II/*blood/genetics
Humans
Male
Middle Aged
Neoplasm Recurrence, Local/blood/*diagnosis
Predictive Value of Tests
*Prostatectomy
Prostatic Neoplasms/blood/*diagnosis/surgery
RNA, Messenger/*blood
Reverse Transcriptase Polymerase Chain Reaction
Severity of Illness Index
Time Factors
Figure
Reference
-
1. Mejean A, Vona G, Nalpas B, Damotte D, Brousse N, Chretien Y, Dufour B, Lacour B, Brechot C, Paterlini-Brechot P. Detection of circulating prostate derived cells in patients with prostate adenocarcinoma is an independent risk factor for tumor recurrence. J Urol. 2000. 163:2022–2029.
Article2. Zippelius A, Pantel K. RT-PCR-based detection of occult disseminated tumor cells in peripheral blood and bone marrow of patients with solid tumors. An overview. Ann N Y Acad Sci. 2000. 906:110–123.
Article3. Moreno JG, Croce CM, Fischer R, Monne M, Vihko P, Mulholland SG, Gomella LG. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992. 52:6110–6112.4. Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993. 53:227–230.5. Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997. 57:2321–2324.6. Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995. 62:552–558.7. Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996. 48:326–334.
Article8. Olsson CA, De Vries GM, Benson MC, Raffo A, Buttyan R, Cama C, O'Toole K, Katz AE. The use of RT-PCR for prostate-specific antigen assay to predict potential surgical failures before radical prostatectomy: molecular staging of prostate cancer. Br J Urol. 1996. 77:411–417.
Article9. Grasso YZ, Gupta MK, Levin HS, Zippe CD, Klein EA. Combined nested RT-PCR assay for prostate-specific antigen and prostate-specific membrane antigen in prostate cancer patients: correlation with pathological stage. Cancer Res. 1998. 58:1456–1459.10. Zhang Y, Zippe CD, Van Lente F, Klein EA, Gupta MK. Combined nested reverse transcription-PCR assay for prostate-specific antigen and prostate-specific membrane antigen in detecting circulating prostatic cells. Clin Cancer Res. 1997. 3:1215–1220.11. Katz AE, Olsson CA, Raffo AJ, Cama C, Perlman H, Seaman E, O'Toole KM, McMahon D, Benson MC, Buttyan R. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology. 1994. 43:765–775.
Article12. Okegawa T, Nutahara K, Higashihara E. Preoperative nested reverse transcription-polymerase chain reaction for prostate specific membrane antigen predicts biochemical recurrence after radical prostatectomy. BJU Int. 1999. 84:112–117.
Article13. Joung JY, Yang SO, Jeong IG, Han KS, Seo HK, Chung J, Park WS, Lee KH. Reverse transcriptase-polymerase chain reaction and immunohistochemical studies for detection of prostate stem cell antigen expression in prostate cancer: potential value in molecular staging of prostate cancer. Int J Urol. 2007. 14:635–643.
Article14. Grossfeld GD, Chang JJ, Broering JM, Li YP, Lubeck DP, Flanders SC, Carroll PR. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy: results from the Cancer of the Prostate Strategic Urologic Research Endeavor database. J Urol. 2001. 165:851–856.
Article15. Donohue JF, Bianco FJ Jr, Kuroiwa K, Vickers AJ, Wheeler TM, Scardino PT, Reuter VA, Eastham JA. Poorly differentiated prostate cancer treated with radical prostatectomy: long-term outcome and incidence of pathological downgrading. J Urol. 2006. 176:991–995.
Article16. Eastham JA, Riedel E, Scardino PT, Shike M, Fleisher M, Schatzkin A, Lanza E, Latkany L, Begg CB. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. JAMA. 2003. 289:2695–2700.17. Su SL, Boynton AL, Holmes EH, Elgamal AA, Murphy GP. Detection of extraprostatic prostate cells utilizing reverse transcription-polymerase chain reaction. Semin Surg Oncol. 2000. 18:17–28.
Article18. Olsson CA, de Vries GM, Buttyan R, Katz AE. Reverse transcriptase-polymerase chain reaction assays for prostate cancer. Urol Clin North Am. 1997. 24:367–378.
Article19. de la Taille A, Olsson CA, Buttyan R, Benson MC, Bagiella E, Cao Y, Burchardt M, Chopin DK, Katz AE. Blood-based reverse transcriptase polymerase chain reaction assays for prostatic specific antigen: long term follow-up confirms the potential utility of this assay in identifying patients more likely to have biochemical recurrence (rising PSA) following radical prostatectomy. Int J Cancer. 1999. 84:360–364.
Article20. Israeli RS, Miller WH Jr, Su SL, Powell CT, Fair WR, Samadi DS, Huryk RF, DeBlasio A, Edwards ET, Wise GJ, Heston WD. Sensitive nested reverse transcription polymerase chain reaction detection of circulating prostatic tumor cells: comparison of prostate-specific membrane antigen and prostate-specific antigen-based assays. Cancer Res. 1994. 54:6306–6310.21. Sokoloff MH, Tso CL, Kaboo R, Nelson S, Ko J, Dorey F, Figlin RA, Pang S, deKernion J, Belldegrun A. Quantitative polymerase chain reaction does not improve preoperative prostate cancer staging: a clinicopathological molecular analysis of 121 patients. J Urol. 1996. 156:1560–1566.
Article22. Loric S, Dumas F, Eschwege P, Blanchet P, Benoit G, Jardin A, Lacour B. Enhanced detection of hematogenous circulating prostatic cells in patients with prostate adenocarcinoma by using nested reverse transcription polymerase chain reaction assay based on prostate-specific membrane antigen. Clin Chem. 1995. 41:1698–1704.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radical Prostatectomy
- The Impact of Positive Surgical Margins on Biochemical Recurrence after Radical Retropubic Prostatectomy
- Predictors of Biochemical Failure after Radical Perineal Prostatectomy for Localized Prostate Cancer
- Cribriform Pattern at the Surgical Margin is Highly Predictive of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy
- How can we best manage biochemical failure after radical prostatectomy?