J Korean Med Sci.
2010 Sep;25(9):1284-1290. 10.3346/jkms.2010.25.9.1284.
Efficacy of Dendritic Cells Matured Early with OK-432 (Picibanil(R)), Prostaglandin E2, and Interferon-alpha as a Vaccine for a Hormone Refractory Prostate Cancer Cell Line
- Affiliations
-
- 1Department of Urology, College of Medicine, Hallym University, Hallym Sacred Heart Hospital, Anyang, Korea.
- 2Department of Urology, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea. cskim@amc.seoul.kr
- 3Department of Urology, Seoul National University, College of Medicine and Seoul National University Hospital Bundang, Seongnam, Korea.
- KMID: 1785906
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1284
Abstract
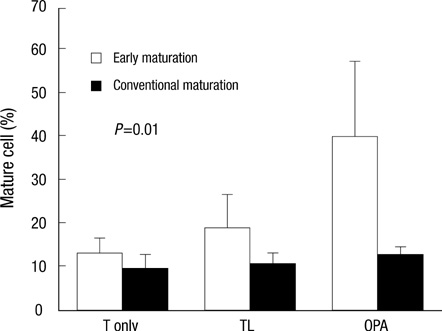
- Dendritic cells (DCs) are potent antigen-presenting cells. OK432 (Picibanil(R)) was introduced as a potent stimulator of DC maturation in combination with prostaglandin-E2 and interferon-alpha. We compared the efficacy of a DC-prostate cancer vaccine using early-mature DCs stimulated with OK432, PGE2 and INF-alpha (OPA) with that of vaccines using other methods. On days 3 or 7 of DC culture, TNF-alpha (T), TNF-alpha and LPS (TL) or OPA were employed as maturation stimulators. DU145 cells subjected to heat stress were hybridized with mature DCs using polyethyleneglycol. T cells were sensitized by the hybrids, and their proliferative and cytokine secretion activities and cytotoxicity were measured. The yields of early-mature DCs were higher, compared to yields at the conventional maturation time (P<0.05). In the early maturation setting, the mean fusion ratios, calculated from the fraction of dual-positive cells, were 13.3%, 18.6%, and 39.9%, respectively (P=0.051) in the T only, TL, and OPA-treated groups. The function of cytotoxic T cells, which were sensitized with the hybrids containing DCs matured early with OPA, was superior to that using other methods. The antitumor effects of DC-DU145 hybrids generated with DCs subjected to early maturation with the OPA may be superior to that of the hybrids using conventional maturation methods.
MeSH Terms
-
Cancer Vaccines/*immunology
Cell Line, Tumor
Dendritic Cells/cytology/drug effects/*immunology
Dinoprostone/*pharmacology
Humans
Immunologic Factors/*pharmacology
Interferon-alpha/*pharmacology
Lipopolysaccharides/toxicity
Male
Neoplasms, Hormone-Dependent/*immunology
Phenotype
Picibanil/*pharmacology
Prostatic Neoplasms/*immunology
T-Lymphocytes, Cytotoxic/immunology
Figure
Reference
-
1. Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997. 186:1183–1187.
Article2. Koido S, Hara E, Homma S, Torii A, Mitsunaga M, Yanagisawa S, Toyama Y, Kawahara H, Watanabe M, Yoshida S, Kobayashi S, Yanaga K, Fujise K, Tajiri H. Streptococcal preparation OK-432 promotes fusion efficiency and enhances induction of antigen-specific CTL by fusions of dendritic cells and colorectal cancer cells. J Immunol. 2007. 178:613–622.
Article3. Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-α as potent immune enhancers. J Immunother. 2006. 29:67–77.
Article4. Koido S, Hara E, Homma S, Mitsunaga M, Takahara A, Nagasaki E, Kawahara H, Watanabe M, Toyama Y, Yanagisawa S, Kobayashi S, Yanaga K, Fujise K, Gong J, Tajiri H. Synergistic induction of antigen-specific CTL by fusion of TLR-stimulated dendritic cells and heat stressed tumor cells. J Immunol. 2007. 179:4874–4883.5. Shu YQ, Gu Y. The effect of dendritic cells activated by OK-432 and pulsed with antigens on cytokine induced killers. Biomed Pharmacother. 2006. 60:156–160.6. Draube A, Beyer M, Schumer S, Thomas RK, von Tresckow B, Koslowsky TC, Krieglstein CF, Schultze JL, Wolf J. Efficient activation of autologous tumor-specific T cells: a simple coculture technique of autologous dendritic cells compared to established cell fusion strategies in primary human colorectal carcinoma. J Immunother. 2007. 30:359–369.
Article7. Larmonier N, Merino D, Nicolas A, Cathelin D, Benson A, Bateman A, Solary E, Martin F, Katsanis E, Bonnotte B. Apoptotic, necrotic, or fused tumor cells: an equivalent source of antigen for dendritic cell loading. Apoptosis. 2006. 11:1513–1524.
Article8. Shimizu K, Kuriyama H, Kjaergaard J, Lee W, Tanaka H, Shu S. Comparative analysis of antigen loading strategies of dendritic cells for tumor immunotheraphy. J Immunother. 2004. 27:265–272.9. Kao JY, Zhang M, Chen CM, Chen JJ. Superior efficacy of dendritic cell-tumor fusion vaccine compared with tumor lysate-pulsed dendritic cell vaccine in colon cancer. Immunol Lett. 2005. 101:154–159.
Article10. Lundqvist A, Palmborg A, Bidla G, Whelan M, Pundha H, Pisa P. Allogeneic tumor-dendritic cell fusion vaccines for generation of broad prostate cancer T-cell responses. Med Oncol. 2004. 21:155–165.
Article11. Tanaka F, Yamaguchi H, Haraguchi N, Mashino K, Ohta M, Inoue H, Mori M. Efficient induction of specific cytotoxic T lymphocytes to tumor rejection peptide using functional matured 2 day-cultured dendritic cells derived from human monocytes. Int J Oncol. 2006. 29:1263–1268.
Article12. Moldenhauer A, Nociari MM, Dias S, Lalezari P, Moore MA. Optimized culture conditions for the generation of dendritic cells from peripheral blood monocytes. Vox Sang. 2003. 84:228–236.
Article13. Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997. 158:2919–2925.14. Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994. 180:1263–1272.
Article15. Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998. 161:3042–3049.16. Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997. 27:3031–3038.
Article17. Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol Scand. 2001. 59:124–130.
Article18. Itoh T, Ueda Y, Okugawa K, Fujiwara H, Fuji N, Yamashita T, Fujiki H, Harada S, Yoshimura T, Yamagishi H. Streptococcal preparation OK432 promotes functional maturation of human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2003. 52:207–214.
Article19. Toyokawa H, Inaba M, Takai S, Satoi S, Beuth J, Ko HL, Matsui Y, Kwon AH, Kamiyama Y, Ikehara S. Enhancement of circulating dendritic cell activity by immunomodulators (OK432 and KP-40). Anticancer Res. 2002. 22:2137–2145.20. Kim KW, Kim SH, Shin JG, Kim GS, Son YO, Park SW, Kwon BH, Kim DW, Lee CH, Sol MY, Jeong MH, Chung BS, Kang CD. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004. 109:685–690.
Article21. Koido S, Hara E, Homma S, Mitsunaga M, Takahara A, Nagasaki E, Kawahara H, Watanabe M, Toyama Y, Yanagisawa S, Kobayashi S, Yanaga K, Fujise K, Gong J, Tajiri H. Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells. J Immunol. 2007. 179:4874–4883.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Establishment of Dendritic Cell-Tumor Fusion Vaccines for Hormone Refractory Prostate Cancer Cell
- Cancer Vaccines
- Glioma Stem Cell-Targeted Dendritic Cells as a Tumor Vaccine Against Malignant Glioma
- Anticancer Efficacy and Toxicity of Oral GMO-paclitaxel in a Hormone Refractory Prostate Cancer Model
- Effect of combination therapy with vinblastine, adriamycin and interferon-alpha, gamma on human renal cell carcinoma cell lines