J Korean Med Sci.
2007 Dec;22(6):1034-1041. 10.3346/jkms.2007.22.6.1034.
Altered Renal Sodium Transporter Expression in an Animal Model of Type 2 Diabetes Mellitus
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Boramae Hospital, Seoul, Korea.
- 2Department of Internal Medicine, College of Medicine, Seoul National University, Seoul, Korea.
- 3Laboratory of Kidney and Electrolyte Metabolism, National Institutes of Health, Bethesda, Maryland, USA.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. kyna@snubh.org
- KMID: 1785766
- DOI: http://doi.org/10.3346/jkms.2007.22.6.1034
Abstract
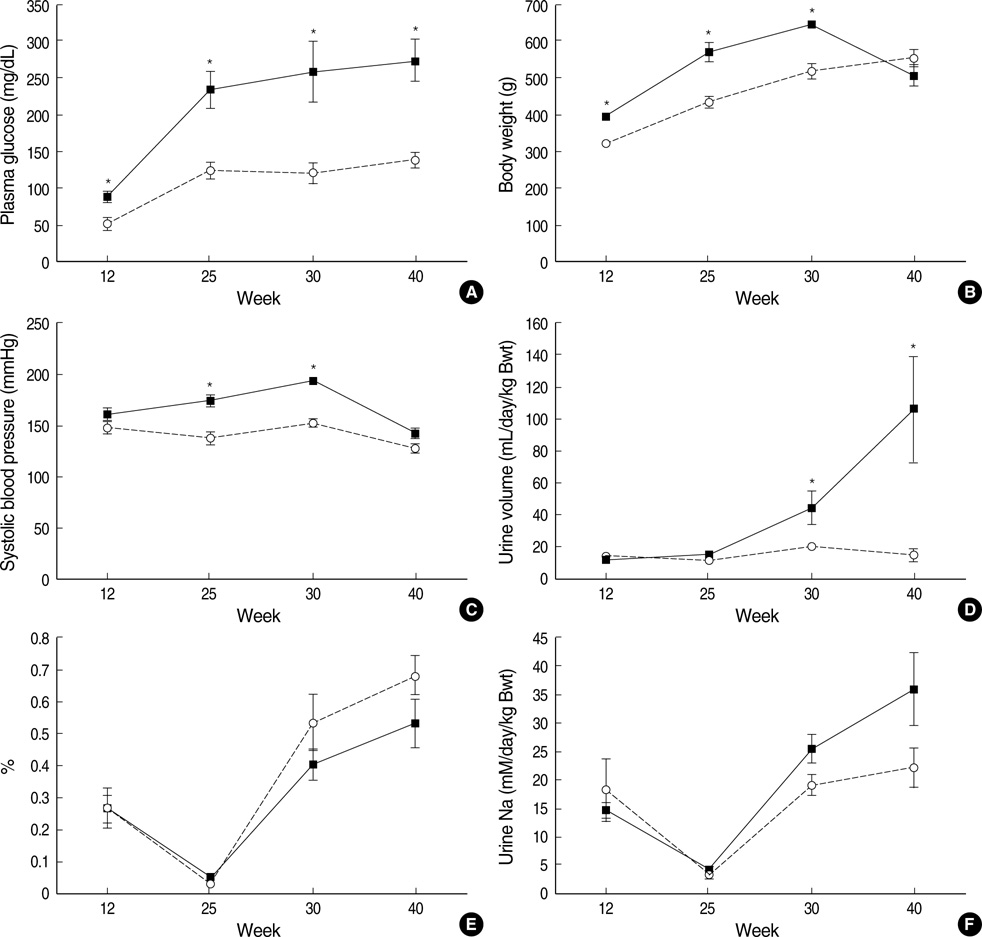
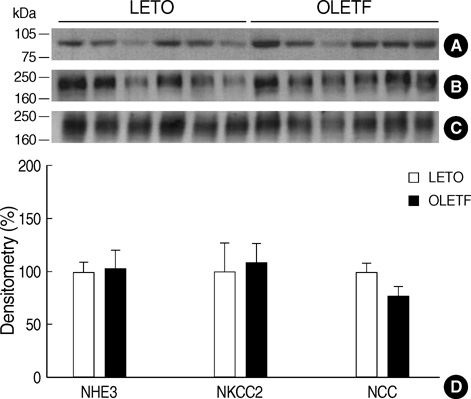
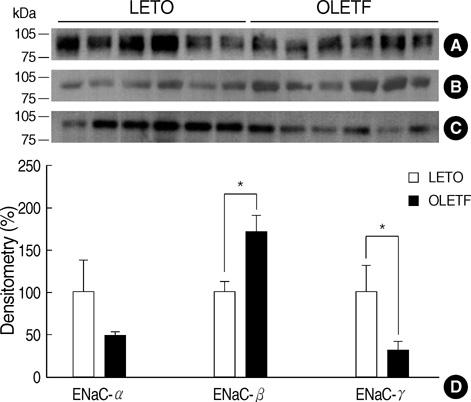
- Hemodynamic factors play an important role in the development and/or progression of diabetic nephropathy. We hypothesized that renal sodium transporter dysregulation might contribute to the hemodynamic alterations in diabetic nephropathy. Otsuka Long Evans Tokushima Fatty (OLETF) rats were used as an animal model for type 2 diabetes. Long Evans Tokushima (LETO) rats were used as controls. Renal sodium transporter regulation was investigated by semiquantitative immunoblotting and immunohistochemistry of the kidneys of 40-week-old animals. The mean serum glucose level in OLETF rats was increased to 235+/-25 mg/dL at 25 weeks, and the hyperglycemia continued up to the end of 40 weeks. Urine protein/ creatinine ratios were 10 times higher in OLETF rats than in LETO rats. At 40th week, the abundance of the epithelial sodium channel (ENaC) beta-subunit was increased in OLETF rats, but the abundance of the ENaC gamma-subunit was decreased. No significant differences were observed in the ENaC alpha-subunit or other major sodium transporters. Immunohistochemistry for the ENaC beta-subunit showed increased immunoreactivity in OLETF rats, whereas the ENaC gamma-subunit showed reduced immunoreactivity in these rats. In OLETF rats, ENaC beta-subunit upregulation and ENaC gamma-subunit downregulation after the development of diabetic nephropathy may reflect an abnormal sodium balance.
Keyword
MeSH Terms
-
Animals
Blood Glucose/analysis
Diabetes Mellitus, Type 2/*metabolism
*Disease Models, Animal
Epithelial Sodium Channel/*analysis
Hypertension/complications
Immunoblotting
Immunohistochemistry
Kidney/*metabolism
Male
Rats
Sodium/*metabolism
Sodium-Hydrogen Antiporter/genetics
Sodium-Potassium-Chloride Symporters/genetics
Figure
Reference
-
1. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.
Article2. O'Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol. 1997. 17:93–100.3. Johnson RJ, Schreiner GF. Hypothesis: the role of acquired tubulointerstitial disease in the pathogenesis of salt-dependent hypertension. Kidney Int. 1997. 52:1169–1179.
Article4. Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002. 346:913–923.
Article5. Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reily R, Igarashi P, Aronson PS. NHE3: an Na+/H+ exchanger isoform of renal brush border. Am J Physiol. 1993. 265:F736–F742.6. Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na(+)-K(+)-2Clcotransporter, BSC-1. Am J Physiol. 1996. 271:F619–F628.7. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998. 95:14552–14557.
Article8. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ, subunit proteins in rat kidney. J Clin Invest. 1999. 104:R19–R23.
Article9. Kim D, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol. 2003. 285:F303–F309.10. Song J, Knepper MA, Verbalis JG, Ecelbarger CA. Increased renal ENaC subunit and sodium transporter abundances in streptozotocin-induced type 1 diabetes. Am J Physiol Renal Physiol. 2003. 285:F1125–F1137.
Article11. Bickel CA, Knepper MA, Verbalis JG, Ecelbarger CA. Dysregulation of renal salt and water transport proteins in diabetic Zucker rats. Kidney Int. 2002. 61:2099–2110.
Article12. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.
Article13. Fukuzawa Y, Watanabe Y, Inaguma D, Hotta N. Evaluation of glomerular lesion and abnormal urinary findings in OLETF rats resulting from a long-term diabetic state. J Lab Clin Med. 1996. 128:568–578.
Article14. Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol. 2001. 281:F639–F648.15. Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(-)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol. 2001. 12:1335–1341.
Article16. Na KY, Oh YK, Han JS, Joo KW, Lee JS, Earm JH, Knepper MA, Kim GH. Upregulation of Na+ transporter abundances in response to chronic thiazide or loop diuretic treatment in rats. Am J Physiol Renal Physiol. 2003. 284:F133–F143.17. Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other renal key sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol. 2006. 290:F1055–F1064.18. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994. 367:463–467.19. Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol. 1998. 274:C1373–C1379.
Article20. Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000. 279:F46–F53.
Article21. Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000. 321:412–419.
Article22. Rossier BC. 1996 Homer Smith Award Lecture. Cum grano salis. The epithelial sodium channel and the control of blood pressure. J Am Soc Nephrol. 1997. 8:980–992.
Article23. Kim SW, Wang W, Kwon TH, Knepper MA, Frøkiaer J, Nielsen S. Increased expression of ENaC subunits and increased apical targeting of AQP2 in the kidneys of spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005. 289:F957–F968.
Article24. Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005. 16:3154–3159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- Letter: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- Paradigm Changes in Diabetes Management Guidelines: American Diabetes Association 2018
- Benefit and Safety of Sodium-Glucose Co-Transporter 2 Inhibitors in Older Patients with Type 2 Diabetes Mellitus
- Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitor