J Vet Sci.
2014 Jun;15(2):289-295. 10.4142/jvs.2014.15.2.289.
Comparable bone healing capacity of different bone graft matrices in a rabbit segmental defect model
- Affiliations
-
- 1Xenotransplantation Research Center, Biomedical Research Institute, Seoul National University Hospital, Seoul 153-832, Korea.
- 2Veterinary Medical Center, Chungbuk National University, Cheongju 361-763, Korea. shchoi@cbnu.ac.kr
- 3College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea.
- KMID: 1784651
- DOI: http://doi.org/10.4142/jvs.2014.15.2.289
Abstract
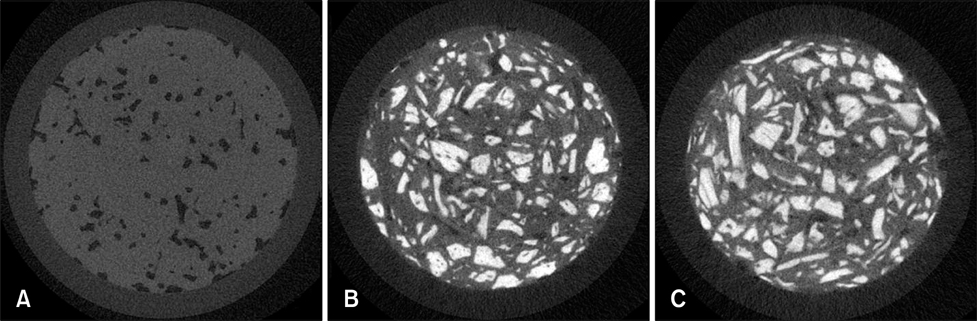
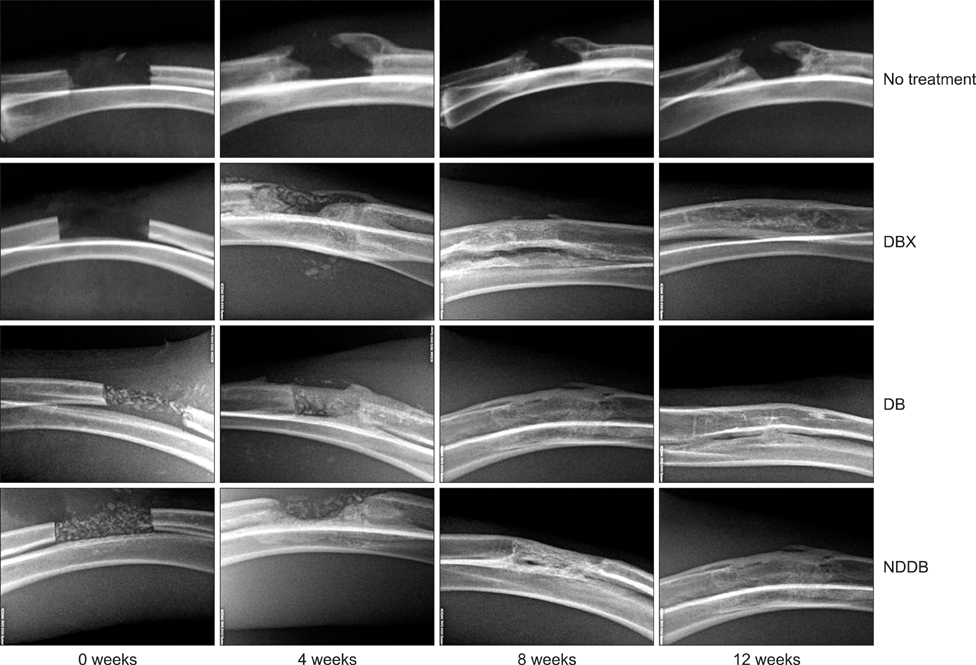
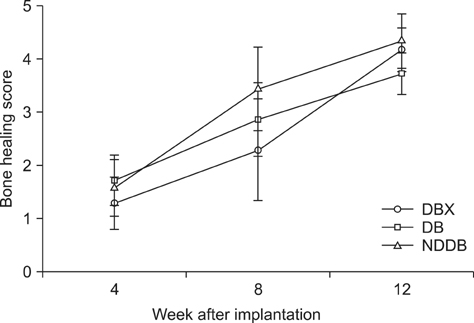
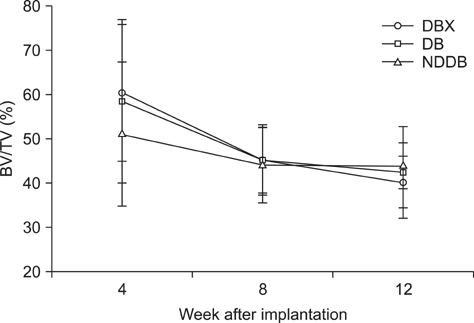
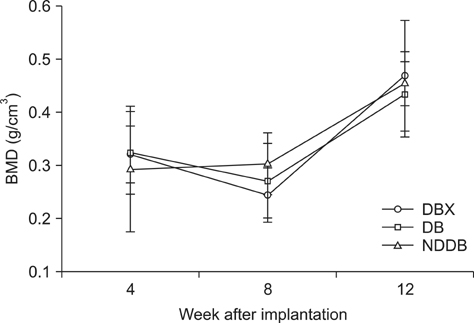
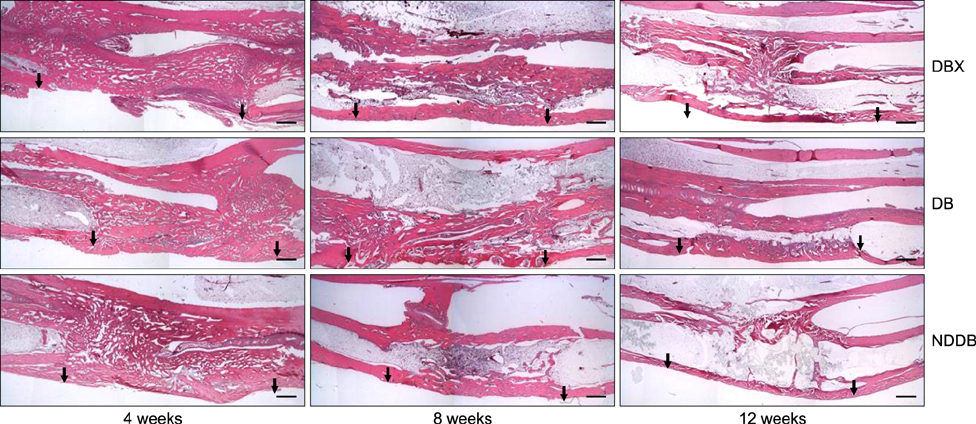
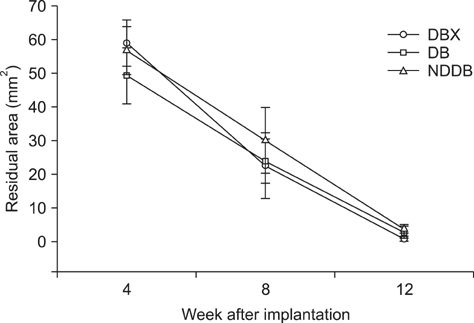
- We compared the bone healing capacity of three different demineralized bone matrix (DBM) products applied using different carrier molecules (hyaluronic acid [HA] vs. carboxymethylcellulose [CMC]) or bone compositions (cortical bone vs. cortical bone and cancellous bone) in a rabbit segmental defect model. Overall, 15-mm segmental defects in the left and right radiuses were created in 36 New Zealand White rabbits and filled with HA-based demineralized cortical bone matrix (DBX), CMC-based demineralized cortical bone matrix (DB) or CMC-based demineralized cortical bone with cancellous bone (NDDB), and the wound area was evaluated at 4, 8, and 12 weeks post-implantation. DBX showed significantly lower radiopacity, bone volume fraction, and bone mineral density than DB and NDDB before implantation. However, bone healing score, bone volume fraction, bone mineral density, and residual bone area at 4, 8, and 12 weeks post-implantation revealed no significant differences in bone healing capacity. Overall, three DBM products with different carrier molecules or bone compositions showed similar bone healing capacity.
MeSH Terms
Figure
Reference
-
1. Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001; 10:Suppl 2. S96–S101.
Article2. Aslan M, Şimşek G, Dayi E. The effect of hyaluronic acid-supplemented bone graft in bone healing: experimental study in rabbits. J Biomater Appl. 2006; 20:209–220.
Article3. Boyan BD, Ranly DM, McMillan J, Sunwoo M, Roche K, Schwartz Z. Osteoinductive ability of human allograft formulations. J Periodontol. 2006; 77:1555–1563.
Article4. Chesmel KD, Branger J, Wertheim H, Scarborough N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J Oral Maxillofac Surg. 1998; 56:857–863.
Article5. Cho BC, Park JW, Baik BS, Kim IS. Clinical application of injectable calcium sulfate on early bony consolidation in distraction osteogenesis for the treatment of craniofacial microsomia. J Craniofac Surg. 2002; 13:465–475.
Article6. Cook SD, Barrack RL, Santman M, Patron LP, Salkeld SL, Whitecloud TS 3rd. Strut allograft healing to the femur with recombinant human osteogenic protein-1. Clin Orthop Relat Res. 2000; 381:47–57.
Article7. Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998; 357:219–228.8. Han B, Tang B, Nimni ME. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res. 2003; 44:160–166.
Article9. Hopp SG, Dahners LE, Gilbert JA. A study of the mechanical strength of long bone defects treated with various bone autograft substitutes: an experimental investigation in the rabbit. J Orthop Res. 1989; 7:579–584.
Article10. Jang CH, Park H, Cho YB, Song CH. Mastoid obliteration using a hyaluronic acid gel to deliver a mesenchymal stem cells-loaded demineralized bone matrix: an experimental study. Int J Pediatr Otorhinolaryngol. 2008; 72:1627–1632.
Article11. Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007; 28:1830–1837.
Article12. Lasa C Jr, Hollinger J, Drohan W, MacPhee M. Delivery of demineralized bone powder by fibrin sealant. Plast Reconstr Surg. 1995; 96:1409–1417.
Article13. Lee YP, Jo M, Luna M, Chien B, Lieberman JR, Wang JC. The efficacy of different commercially available demineralized bone matrix substances in an athymic rat model. J Spinal Disord Tech. 2005; 18:439–444.
Article14. Leupold JA, Barfield WR, An YH, Hartsock LA. A comparison of ProOsteon, DBX, and collagraft in a rabbit model. J Biomed Mater Res B Appl Biomater. 2006; 79:292–297.
Article15. Matzenbacher SA, Mailhot JM, McPherson JC 3rd, Cuenin MF, Hokett SD, Sharawy M, Peacock ME. In vivo effectiveness of a glycerol-compounded demineralized freeze-dried bone xenograft in the rat calvarium. J Periodontol. 2003; 74:1641–1646.
Article16. Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine. 1998; 23:159–167.
Article17. Oakes DA, Lee CC, Lieberman JR. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop Relat Res. 2003; 413:281–290.
Article18. Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of commercially available demineralized bone matrix: preparations in a spine fusion model. J Bone Joint Surg Am. 2004; 86:2243–2250.19. Pinholt EM, Solheim E, Bang G, Sudmann E. Bone induction by composite of bioerodible polyorthoester and demineralized bone matrix in rats. Acta Orthop Scand. 1991; 62:476–480.
Article20. Reynolds MA, Aichelmann-Reidy ME, Kassolis JD, Prasad HS, Rohrer MD. Calcium sulfate-carboxymethylcellulose bone graft binder: histologic and morphometric evaluation in a critical size defect. J Biomed Mater Res B Appl Biomater. 2007; 83:451–458.
Article21. Takahashi Y, Yamamoto M, Yamada K, Kawakami O, Tabata Y. Skull bone regeneration in nonhuman primates by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2007; 13:293–300.
Article22. Turner TM, Urban RM, Hall DJ, Infanger S, Gitelis S, Petersen DW, Haggard WO. Osseous healing using injectable calcium sulfate-based putty for the delivery of demineralized bone matrix and cancellous bone chips. Orthopedics. 2003; 26:5 Suppl. s571–s575.
Article23. Wang H, Springer ING, Schildberg H, Acil Y, Ludwig K, Rueger DR, Terheyden H. Carboxymethylcellulose-stabilized collagenous rhOP-1 device-a novel carrier biomaterial for the repair of mandibular continuity defects. J Biomed Mater Res A. 2004; 68:219–226.
Article24. Wang JC, Alanay A, Mark D, Kanim LEA, Campbell PA, Dawson EG, Lieberman JR. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007; 16:1233–1240.
Article25. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989; 3:192–195.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autogenous Living Bone Graft and Dead Bone Graft in the Rabbit Tibia
- Bone healing capacity of the new fluoridated hydroxyapatite in the rabbit cranium defect
- A comparative study of various type of autogeneous bone graft on the rabbit-skull defect healing
- An experimental study for the evaluation of bone healing capacity of autogenous bone marrow and fibrin composite graft on ulnardiaphyseal bone defect
- Bone regeneration of the fluoridated hydroxyapatite and the bio-glass in the rabbit cranium defect model