J Korean Med Sci.
2009 Dec;24(6):1051-1057. 10.3346/jkms.2009.24.6.1051.
Hypocapnia Attenuates, and Nitrous Oxide Disturbs the Cerebral Oximetric Response to the Rapid Introduction of Desflurane
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, and Medical Research Institute, Dongguk University College of Medicine, Goyang, Korea. ylee@dongguk.ac.kr
- KMID: 1783132
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1051
Abstract
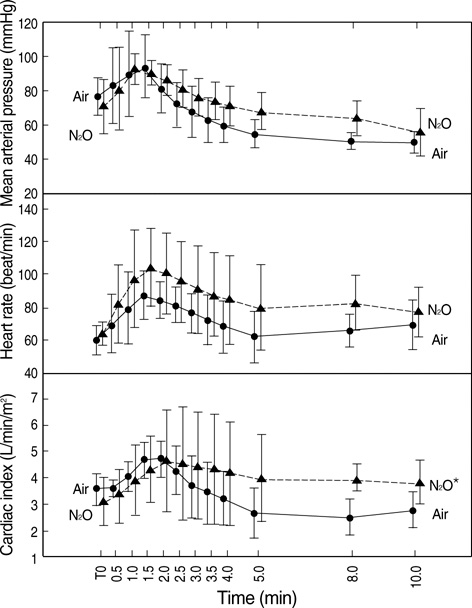
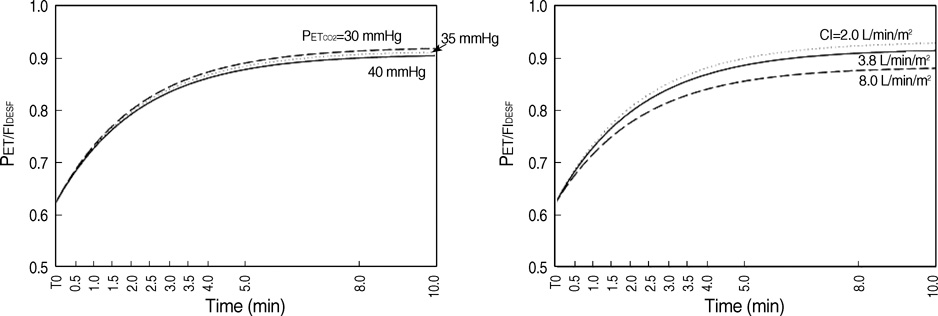
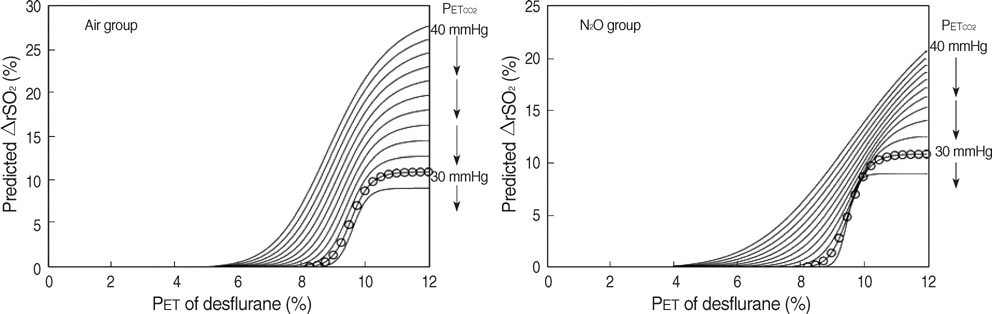
- The aim of this study was to develop a nonlinear mixed-effects model for the increase in cerebral oximetry (rSO2) during the rapid introduction of desflurane, and to determine the effect of hypocapnia and N2O on the model. Twelve American Society of Anesthesiologist physical status class 1 and 2 subjects were allocated randomly into an Air and N2O group. After inducing anesthesia, desflurane was then increased abruptly from 4.0 to 12.0%. The PET(CO2), PET(DESF) and rSO2 were recorded at 12 predetermined periods for the following 10 min. The maximum increase in rSO2 reached +24-25% during normocapnia. The increase in rSO2 could be fitted to a four parameter logistic equation as a function of the logarithm of PET(DESF). Hypocapnia reduced the maximum response of rSO2, shifted the EC50 to the right, and increased the slope in the Air group. N2O shifted the EC50 to the right, and reduced the slope leaving the maximum rSO2 unchanged. The N2O-effects disappeared during hypocapnia. The cerebrovascular reactivity of rSO2 to CO2 is still preserved during the rapid introduction of desflurane. N2O slows the response of rSO2. Hypocapnia overwhelms all the effects of N2O.
Keyword
MeSH Terms
-
Adult
Anesthetics, Inhalation/*pharmacology
*Cerebral Cortex/blood supply/drug effects/physiology
Cerebrovascular Circulation/*drug effects/physiology
Female
Hemodynamics
Humans
Hypocapnia/*metabolism
Isoflurane/*analogs & derivatives/pharmacology
Male
Middle Aged
Models, Theoretical
Nitrous Oxide/*metabolism
*Oximetry
Random Allocation
Regional Blood Flow/drug effects
Figure
Reference
-
1. Ebert TJ, Muzi M, Lopatka CW. Neurocirculatory responses to sevoflurane in humans. A comparison to desflurane. Anesthesiology. 1995. 83:88–95.2. Ebert TJ, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers. A comparison with isoflurane. Anesthesiology. 1993. 79:444–453.3. Daniel M, Larson MD, Eger EI 2nd, Noorani M, Weiskopf RB. Fentanyl, clonidine, and repeated increases in desflurane concentration, but not nitrous oxide or esmolol, block the transient mydriasis caused by rapid increases in desflurane concentration. Anesth Analg. 1995. 81:372–378.
Article4. Brenet O, Granry JC, Poirier N, Le Gall R. The effect of desflurane on cerebral blood flow velocity and cerebrovascular reactivity to CO2 in children. Ann Fr Anesth Reanim. 1998. 17:227–233.5. Tonner PH, Scholz J, Krause T, Paris A, von Knobelsdorff G, Schulte an Esch J. Administration of sufentanil and nitrous oxide blunts cardiovascular effects of desflurane but does not prevent an increase in middle cerebral artery blood flow velocity. Eur J Anaesthesiol. 1997. 14:389–396.
Article6. Hoffman WE, Charbel FT, Edelman G. Desflurane increases brain tissue oxygenation and pH. Acta Anaesthesiol Scand. 1997. 41:1162–1166.
Article7. Mielck F, Stephan H, Buhre W, Weyland A, Sonntag H. Effects of 1 MAC desflurane on cerebral metabolism, blood flow and carbon dioxide reactivity in humans. Br J Anaesth. 1998. 81:155–160.
Article8. Luginbuehl IA, Karsli C, Bissonnette B. Cerebrovascular reactivity to carbon dioxide is preserved during hypocapnia in children anesthetized with 1.0 MAC, but not with 1.5 MAC desflurane. Can J Anaesth. 2003. 50:166–171.
Article9. Lee YS, Kwon TM, In JY, Woo SH, Yon JH, Kim JW, Choe WJ, Kim KM, Hong KH. Reliability of rSO2 to measure CO2 reactivity of cerebral vasculatures during desflurane-N2O anesthesia. Korean J Anesthesiol. 2002. 43:288–293.10. Girling KJ, Cavill G, Mahajan RP. The effects of nitrous oxide and oxygen on transient hyperemic response in human volunteers. Anesth Analg. 1999. 89:175–180.
Article11. Reinstrup P, Ryding E, Algotsson L, Berntman L, Uski T. Regional cerebral blood flow (SPECT) during anaesthesia with isoflurane and nitrous oxide in humans. Br J Anaesth. 1997. 78:407–411.
Article12. Kaisti KK, Langsjo JW, Aalto S, Oikonen V, Sipila H, Teras M, Hinkka S, Metsahonkala L, Scheinin H. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003. 99:603–613.
Article13. Manohar M, Parks C. Regional distribution of brain and myocardial perfusion in swine while awake and during 1.0 and 1.5 MAC isoflurane anaesthesia produced without or with 50% nitrous oxide. Cardiovasc Res. 1984. 18:344–353.
Article14. Cho S, Fujigaki T, Uchiyama Y, Fukusaki M, Shibata O, Sumikawa K. Effects of sevoflurane with and without nitrous oxide on human cerebral circulation. Transcranial Doppler study. Anesthesiology. 1996. 85:755–760.15. Reinstrup P, Ryding E, Ohlsson T, Dahm PL, Uski T. Cerebral blood volume (CBV) in humans during normo- and hypocapnia: influence of nitrous oxide (N2O). Anesthesiology. 2001. 95:1079–1082.16. Karsli C, Luginbuehl IA, Bissonnette B. The effect of nitrous oxide on cerebral blood flow velocity in children anaesthetised with desflurane. Anaesthesia. 2003. 58:24–27.
Article17. Manohar M. Regional distribution of porcine brain blood flow during 50% nitrous oxide administration. Am J Vet Res. 1985. 46:831–835.18. Kannurpatti SS, Biswal BB, Kim YR, Rosen BR. Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. Neuroimage. 2008. 40:1738–1747.
Article19. Weiskopf RB, Eger EI 2nd, Noorani M, Daniel M. Repetitive rapid increases in desflurane concentration blunt transient cardiovascular stimulation in humans. Anesthesiology. 1994. 81:843–849.
Article20. Everitt B, Rabe-Hesketh S. Analyzing Medical Data Using S-PLUS. 2001. New York: Springer-Verlag.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response of cerebral oximetry to increase in alveolar concentration of desflurane: effect of remifentanil and cerebrovascular CO2 reactivity
- Effects of Nitrous Oxide and Desflurane on Cardiovascular Responses to Endotracheal Intubation
- Effects of Nitrous Oxide and Remifentanil on Cardiovascular Response to Endotracheal Intubation during Desflurane Anesthesia
- The Effect of Continuous Infusion of Alfentanil in Combination with Desflurane
- Effect of Nitrous Oxide on Bispectral Index during Sevoflurane Anesthesia