Yonsei Med J.
2009 Feb;50(1):12-21. 10.3349/ymj.2009.50.1.12.
Intracellular Bacterial Infection and Invariant NKT Cells
- Affiliations
-
- 1Laboratory of Immunology, Department of Laboratory Sciences, Gunma University School of Health Sciences, Maebashi, Gunma, Japan. memoto@health.gunma-u.ac.jp
- KMID: 1782962
- DOI: http://doi.org/10.3349/ymj.2009.50.1.12
Abstract
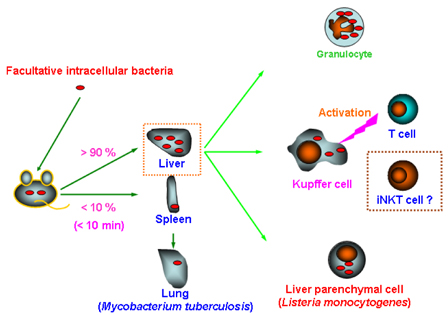
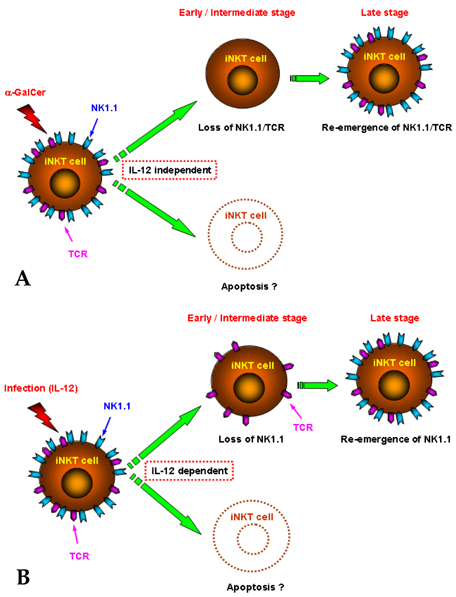
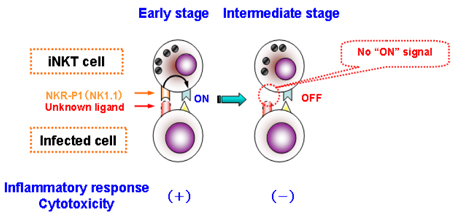
- The invariant (i) natural killer (NK)T cells represent a unique subset of T lymphocytes which express the V alpha14 chain of the T cell receptor (TCR), that recognizes glycolipid antigens presented by the nonpolymorphic major histocompatibility complex (MHC) class I-like antigen presentation molecule CD1d, and they participate in protection against some microbial pathogens. Although iNKT cells have originally been regarded as T cells co-expressing NKR-P1B/C (NK1.1: CD 161), they do not seem to consistently express this marker, since NK1.1 surface expression on iNKT cells undergoes dramatic changes following facultative intracellular bacterial infection, which is correlated with functional changes of this cell population. Accumulating evidence suggests that NK1.1 allows recognition of "missing-self", thus controling activation/inhibition of NK1.1-expressing cells. Therefore, it is tempting to suggest that iNKT cells participate in the regulation of host immune responses during facultative intracellular bacterial infection by controlling NK1.1 surface expression. These findings shed light not only on the unique role of iNKT cells in microbial infection, but also provide evidence for new aspects of the NK1.1 as a regulatory molecule on these cells.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Co-Immunization of Plasmid DNA Encoding IL-12 and IL-18 with Bacillus Calmette-Guérin Vaccine against Progressive Tuberculosis
Bo-Young Jeon, Hyungjin Eoh, Sang-Jun Ha, Hyeeun Bang, Seung-Cheol Kim, Young-Chul Sung, Sang-Nae Cho
Yonsei Med J. 2011;52(6):1008-1015. doi: 10.3349/ymj.2011.52.6.1008.
Reference
-
1. Kaufmann SH. Paul WE, editor. Immunity to intracellular bacteria. Fundamental Immunology. 2003. 5th ed. Philadelphia: Lippincott-Raven Publishers;1229–1261.2. North RJ, Conlan JW. Immunity to Listeria monocytogenes. Chem Immunol. 1998. 70:1–20.3. Gregory SH, Wing EJ. Neutrophil-Kupffer-cell interaction in host defenses to systemic infections. Immunol Today. 1998. 19:507–510.
Article4. Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003. 24:364–369.
Article5. Emoto M, Emoto Y, Kaufmann SH. IL-4 producing CD4+ TCRαβint liver lymphocytes: influence of thymus, β2-microglobulin and NK1.1 expression. Int Immunol. 1995. 7:1729–1739.
Article6. Emoto M, Emoto Y, Kaufmann SH. Interleukin-4-producing CD4+ NK1.1+ TCRαβintermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur J Immunol. 1995. 25:3321–3325.
Article7. Emoto M, Emoto Y, Buchwalow IB, Kaufmann SH. Induction of IFN-γ-producing CD4+ natural killer T cells by Mycobacterium bovis bacillus Calmette Guérin. Eur J Immunol. 1999. 29:650–659.
Article8. Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997. 278:1623–1626.
Article9. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998. 95:5690–5693.
Article10. Falcone M, Yeung B, Tucker L, Rodriguez E, Sarvetnick N. A defect in interleukin 12-induced activation and interferon γ secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus. J Exp Med. 1999. 190:963–972.
Article11. Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001. 413:531–534.
Article12. Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001. 7:1052–1056.
Article13. Denkers EY, Scharton-Kerston T, Barbieri S, Caspar P, Sher A. A role for CD4+NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996. 184:131–139.
Article14. Ishikawa H, Hisaeda H, Taniguchi M, Nakayama T, Sakai T, Maekawa Y, et al. CD4+ Vα14 NKT cells play a crucial role in an early stage of protective immunity against infection with Leishmania major. Int Immunol. 2000. 12:1267–1274.
Article15. Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. α-galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci U S A. 2000. 97:8461–8466.
Article16. Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, et al. Activation of Vα14+ natural killer T cells by α-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001. 69:213–220.
Article17. Exley MA, Bigley NJ, Cheng O, Tahir SM, Smiley ST, Carter QL, et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001. 69:713–718.18. Duthie MS, Wleklinski-Lee M, Smith S, Nakayama T, Taniguchi M, Kahn SJ. During Trypanosoma cruzi infection CD1-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect Immun. 2002. 70:36–48.
Article19. Johnson TR, Hong S, van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002. 76:4294–4303.
Article20. Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002. 8:588–593.21. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003. 4:1230–1237.
Article22. Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα14-Jα281 TCR. J Immunol. 2003. 170:1430–1434.
Article23. Kawakami K, Yamamoto N, Kinjo Y, Miyagi K, Nakasone C, Uezu K, et al. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003. 33:3322–3330.24. Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004. 200:895–904.
Article25. Smiley ST, Lanthier PA, Couper KN, Szaba FM, Boyson JE, Chen W, et al. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J Immunol. 2005. 174:7904–7911.26. Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999. 189:1973–1980.27. Teixeira HC, Kaufmann SH. Role of NK1.1+ cells in experimental listeriosis. NK1+ cells are early IFN-γ producers but impair resistance to Listeria monocytogenes infection. J Immunol. 1994. 152:1873–1882.28. Emoto M, Yoshizawa I, Emoto Y, Miamoto M, Hurwitz R, Kaufmann SH. Rapid development of a gamma interferon-secreting glycolipid/CD1d-specific Vα14+NK 1.1- T-cell subset after bacterial infection. Infect Immun. 2006. 74:5903–5913.
Article29. Szalay G, Ladel CH, Blum C, Brossay L, Kronenberg M, Kaufmann SH. Cutting edge: anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-β2 and Th1 cytokines and ameliorates listeriosis in mice. J Immunol. 1999. 162:6955–6958.30. Kawakami K, Kinjo Y, Uezu K, Yara S, Miyagi K, Koguchi Y, et al. Minimal contribution of Vα14 natural killer T cells to Th1 response and host resistance against mycobacterial infection in mice. Microbiol Immunol. 2002. 46:207–210.
Article31. Bilenki L, Wang S, Yang J, Fan Y, Joyee AG, Yang X. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol. 2005. 175:3197–3206.
Article32. Cornish AL, Keating R, Kyparissoudis K, Smyth MJ, Carbone FR, Godfrey DI. NKT cells are not critical for HSV-1 disease resolution. Immunol Cell Biol. 2006. 84:13–19.
Article33. Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996. 183:2391–2396.
Article34. Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRγ is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997. 186:1957–1963.35. Ryan JC, Seaman WE. Divergent functions of lectin-like receptors on NK cells. Immunol Rev. 1997. 155:79–89.
Article36. Carlyle JR, Martin A, Mehra A, Attisano L, Tsui FW, Zúñiga-Pflücker JC. Mouse NKR-P1B, a novel NK1.1 antigen with inhibitory function. J Immunol. 1999. 162:5917–5923.37. Kung SK, Su RC, Shannon J, Miller RG. The NKR-P1B gene product is an inhibitory receptor on SJL/J NK cells. J Immunol. 1999. 162:5876–5887.38. Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKR-P1 family of natural killer cell receptors. Nat Immunol. 2003. 4:801–807.
Article39. Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci U S A. 2004. 101:3527–3532.
Article40. Aldemir H, Prod'homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, et al. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005. 175:7791–7795.41. Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000. 191:1895–1903.42. Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000. 192:741–754.43. Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A Natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1-CD4+ CD1d-dependent precursor stage. J Exp Med. 2002. 195:835–844.44. Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002. 296:553–555.
Article45. Emoto M, Emoto Y, Kaufmann SH. Bacille Calmette Guérin and interleukin-12 down-modulate interleukin-4-producing CD4+NK1+ T lymphocytes. Eur J Immunol. 1997. 27:183–188.
Article46. Emoto Y, Emoto M, Kaufmann SH. Transient control of interleukin-4-producing natural killer T cells in the livers of Listeria monocytogenes-infected mice by interleukin-12. Infect Immun. 1997. 65:5003–5009.47. Chen H, Huang H, Paul WE. NK1.1+ CD4+ T cells lose NK11 expression upon in vitro activation. J Immunol. 1997. 158:5112–5119.48. Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998. 9:345–353.
Article49. Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000. 30:985–992.50. Osman Y, Kawamura T, Naito T, Takeda K, van Kaer L, Okumura K, et al. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000. 30:1919–1928.
Article51. Leite-de-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J Immunol. 2000. 165:4367–4371.
Article52. Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001. 194:29–44.
Article53. Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, et al. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001. 75:10746–10754.
Article54. Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002. 3:867–874.
Article55. Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002. 169:4450–4459.
Article56. Motsinger A, Haas DW, Stanic AK, van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002. 195:869–879.
Article57. Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003. 171:4020–4027.
Article58. Wilson MT, Johansson C, Olivares-Villagómez D, Singh AK, Stanic AK, Wang C, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003. 100:10913–10918.
Article59. Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, et al. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int Immunol. 2004. 16:241–247.
Article60. Berntman E, Rolf J, Johansson C, Anderson P, Cardell SL. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur J Immunol. 2005. 35:2100–2109.
Article61. Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur J Immunol. 2005. 35:879–889.
Article62. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003. 3:133–146.
Article63. Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-γ. J Immunol. 1994. 152:1883–1887.64. Emoto M, Yoshizawa I, Emoto Y, Takahashi Y, Hurwitz R, Miamoto M, et al. Reversible NK1.1 surface expression by invariant liver natural killer T cells during Listeria monocytogenes infection. Microbes Infect. 2007. 9:1511–1520.
Article65. Emoto Y, Yoshizawa I, Hurwitz R, Brinkmann V, Kaufmann SH, Emoto M. Role of interleukin-12 in determining differential kinetics of invariant natural killer T cells in response to differential burden of Listeria monocytogenes. Microbes Infect. 2008. 10:224–232.
Article66. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000. 13:715–725.
Article67. Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999. 162:6410–6419.68. Legendre V, Boyer C, Guerder S, Arnold B, Hämmerling G, Schmitt-Verhulst AM. Selection of phenotypically distinct NK1.1+ T cells upon antigen expression in the thymus or in the liver. Eur J Immunol. 1999. 29:2330–2343.
Article69. Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999. 29:3768–3781.
Article70. Emoto M, Zerrahn J, Miyamoto M, Pérarnau B, Kaufmann SH. Phenotypic characterization of CD8+NKT cells. Eur J Immunol. 2000. 30:2300–2311.71. Coles MC, McMahon CW, Takizawa H, Raulet DH. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur J Immunol. 2000. 30:236–244.
Article72. Koyasu S. CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both α-βTCR+CD4-CD8- and γ-δ TCR+CD4-CD8-cells. J Exp Med. 1994. 179:1957–1972.73. Arase H, Ono S, Arase N, Park SY, Wakizaka K, Watanabe H, et al. Developmental arrest of NK1.1+ T cell antigen receptor (TCR)-α/β+ T cells and expansion of NK1.1+ TCR-γ/δ+ T cell development in CD3ζ-deficient mice. J Exp Med. 1995. 182:891–895.
Article74. Vicari AP, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+NK1.1+ thymocytes. Eur J Immunol. 1996. 26:1424–1429.
Article75. Emoto M, Miyamoto M, Emoto Y, Zerrahn J, Kaufmann SH. A critical role of T-cell receptor γ/δ cells in antibacterial protection in mice early in life. Hepatology. 2001. 33:887–893.
Article76. Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, et al. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002. 3:966–974.
Article77. Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003. 100:2663–2668.
Article78. Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J Immunol. 1997. 159:5931–5935.79. Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995. 182:2091–2096.
Article80. Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998. 10:1491–1499.81. Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur J Immunol. 1999. 29:3313–3318.82. Kameyama H, Kawamura T, Naito T, Bannai M, Shimamura K, Hatakeyama K, et al. Size of the population of CD4+ natural killer T cells in the liver is maintained without supply by the thymus during adult life. Immunology. 2001. 104:135–141.
Article83. Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997. 15:535–562.
Article84. Davodeau F, Peyrat MA, Necker A, Dominici R, Blanchard F, Leget C, et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J Immunol. 1997. 158:5603–5611.85. Hameg A, Apostolou I, Leite-De-Moraes M, Gombert JM, Garcia C, Koezuka Y, et al. A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the α-galactosylceramide antigen. J Immunol. 2000. 165:4917–4926.
Article86. Takahashi T, Nieda M, Koezuka Y, Nicol A, Porcelli SA, Ishikawa Y, et al. Analysis of human Vα24+CD4+ NKT cells activated by α-glycosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2000. 164:4458–4464.87. Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002. 195:625–636.
Article88. Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002. 195:637–641.
Article89. Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003. 100:8395–8400.
Article90. Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003. 198:1069–1076.
Article91. Plougastel B, Matsumoto K, Dubbelde C, Yokoyama WM. Analysis of a 1-Mb BAC contig overlapping the mouse Nkrp1 cluster of genes: cloning of three new Nkrp1 members, Nkrp1d, Nkrp1e, and Nkrp1f. Immunogenetics. 2001. 53:592–598.
Article92. Holmberg LA, Ault KA. Characterization of Listeria monocytogenes-induced murine natural killer cells. Immunol Res. 1986. 5:50–60.
Article93. Bancroft GJ, Schreiber RD, Unanue ER. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991. 124:5–24.
Article94. Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, et al. Immune response in mice that lack the interferon-γ receptor. Science. 1993. 259:1742–1745.
Article95. Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN γ. Immunity. 1995. 3:109–117.
Article96. Iizawa Y, Czuprynski C. Effects of administration of murine recombinant IL-4 on the resistance of mice to Listeria monocytogenes infection. Immunol Lett. 1992. 32:185–189.
Article97. Haak-Frendscho M, Brown JF, Iizawa Y, Wagner RD, Czuprynski CJ. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992. 148:3978–3985.98. Szalay G, Ladel CH, Blum C, Kaufmann SH. IL-4 neutralization or TNF-γ treatment ameliorate disease by an intracellular pathogen in IFN-γ receptor-deficient mice. J Immunol. 1996. 157:4746–4750.99. Kaufmann SH, Emoto M, Szalay G, Barsig J, Flesch IE. Interleukin-4 and listeriosis. Immunol Rev. 1997. 158:95–105.
Article100. Ranson T, Bregenholt S, Lehuen A, Gaillot O, Leite-de-Moraes MC, Herbelin A, et al. Invariant Vα14+ NKT cells participate in the early response to enteric Listeria monocytogenes infection. J Immunol. 2005. 175:1137–1144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural killer T cell and pathophysiology of asthma
- Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Acute Cholecystitis
- B Cells Promote Th1- Skewed NKT Cell Response by CD1d-TCR Interaction
- Comparison of Invariant NKT Cells with Conventional T Cells by Using Gene Set Enrichment Analysis (GSEA)
- Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Multiple Trauma