J Korean Med Sci.
2011 Feb;26(2):222-230. 10.3346/jkms.2011.26.2.222.
Effects of Benzo(a)pyrene on the Expression of Heat Shock Proteins, Pro-inflammatory Cytokines and Antioxidant Enzymes in Hepatic Tumors Induced by Rat Hepatoma N1-S1 Cells
- Affiliations
-
- 1Graduate School of Medicine, Korea University, Seoul, Korea. dsul@korea.ac.kr
- 2Department of Nanobiomedical Science, College of Advanced Science, Dankook University, Cheonan, Korea.
- 3Department of Pathology, College of Medicine, Korea University, Seoul, Korea.
- 4School of Biological Sciences and Technology, Chonnam National University, Gwangju, Korea.
- 5Environmental Toxico-Genomic and Proteomic Center, College of Medicine, Korea University, Seoul, Korea.
- KMID: 1782110
- DOI: http://doi.org/10.3346/jkms.2011.26.2.222
Abstract
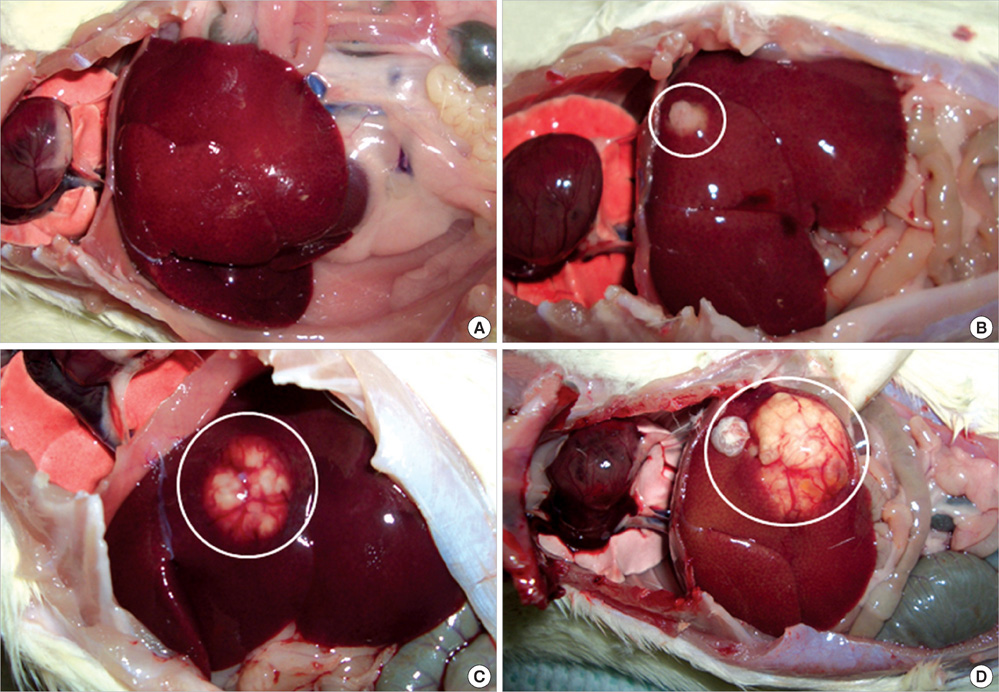
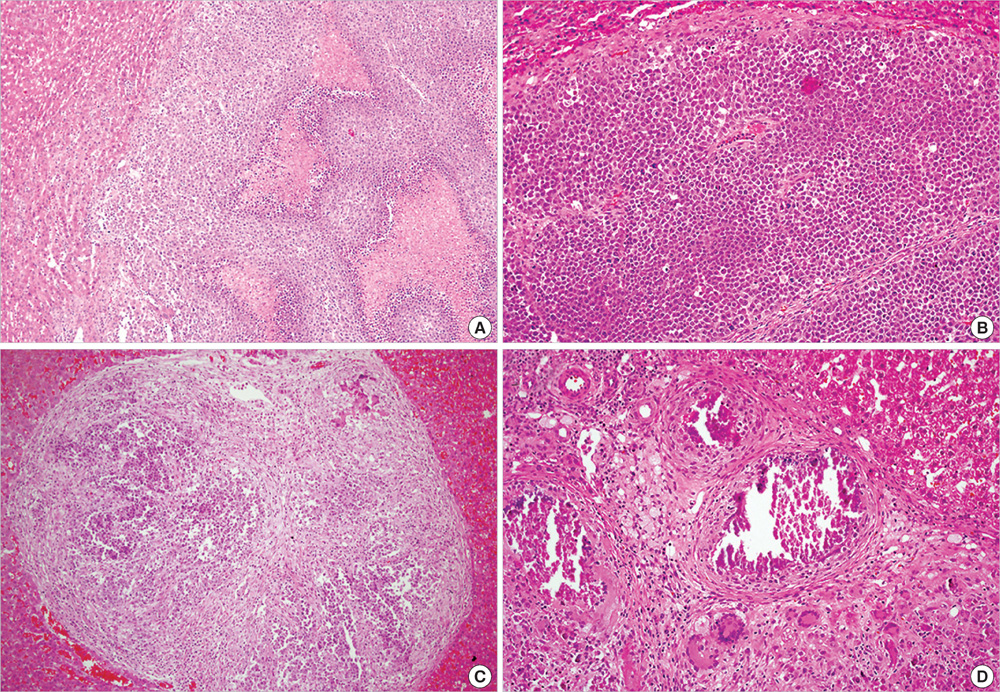
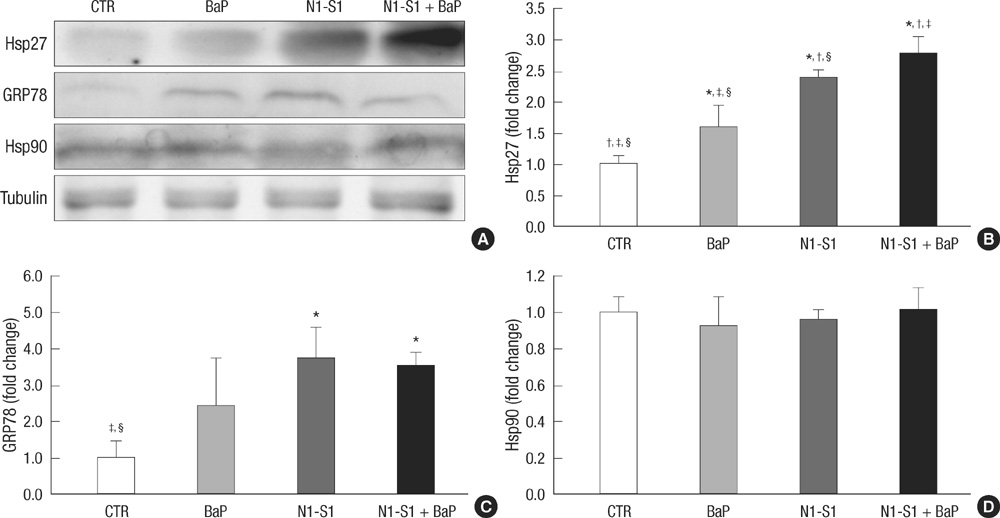
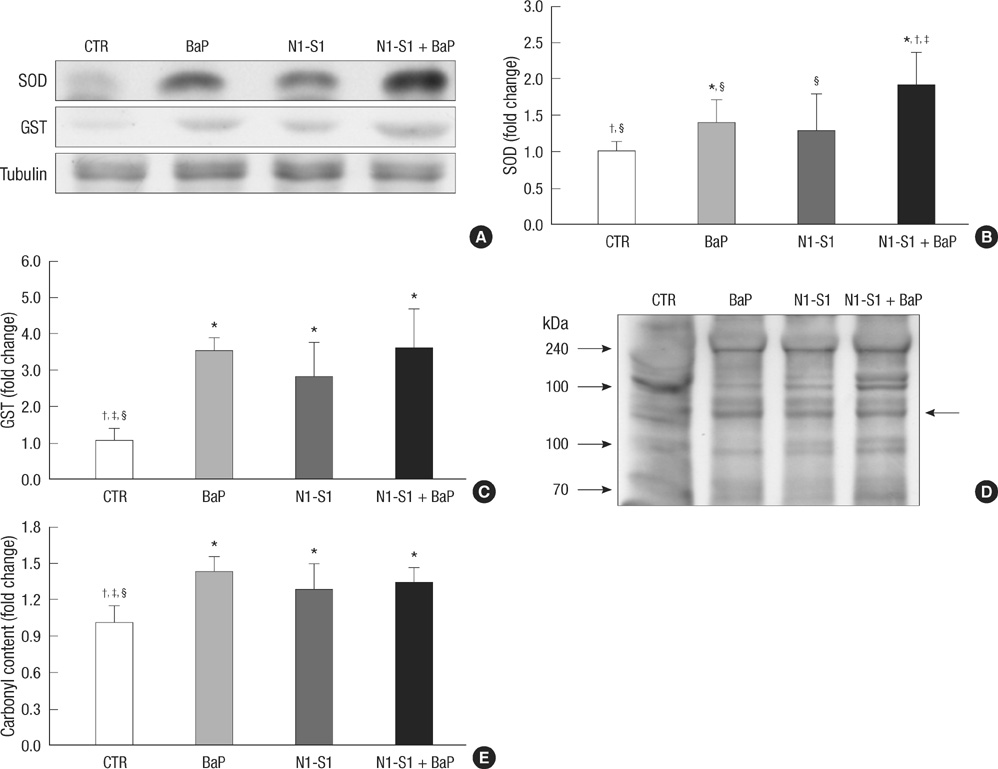
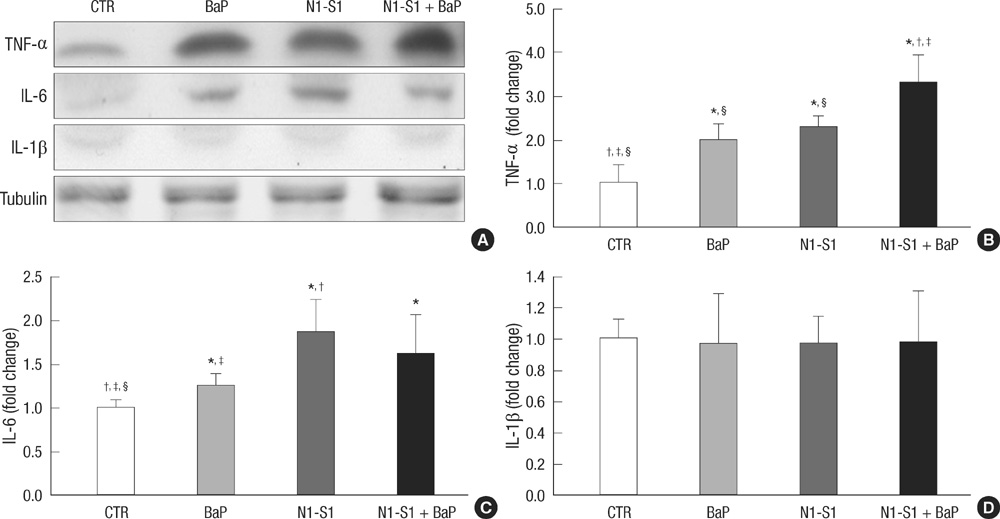
- Benzo(a)pyrene (BaP) is a polycyclic aromatic hydrocarbon (PAH) that is easily introduced to humans via consumption of grilled or smoked meat. BaP causes harmful oxidative effects on cell development, growth and survival through an increase in membrane lipid peroxidation, oxidative DNA damage and mutagenesis. Therefore, the present study was conducted to evaluate the synergistic effects of BaP on oxidative stress in hepatic tumors. In this study, we established a hepatic tumor model by injecting rat hepatoma N1-S1 cells into healthy rats. Changes in the abundance of heat shock proteins (HSPs), antioxidant enzymes and pro-inflammatory cytokines were then investigated by western blot analysis. In addition, we examined changes in oxidative stress levels. Injection of N1-S1 cells or concomitant injection of BaP and N1-S1 cells resulted in the formation of hepatic tumors at the injection site. Evaluation of rat plasma reveals that hepatic tumors induced by BaP and N1-S1 cells expresses higher levels of Hsp27, superoxide dismutase (SOD), and tumor necrosis factor-alpha (TNF-alpha) when compared to those induced by N1-S1 cells only. The collective results of this study suggest that BaP exerts synergistic effects on the expression of HSP, cytokines and antioxidant enzymes in hepatic tumors induced by rat hepatoma N1-S1 cells.
Keyword
MeSH Terms
-
Animals
Antioxidants/*metabolism
Benzo(a)pyrene/*pharmacology
Carcinoma, Hepatocellular/metabolism/pathology
Cell Line, Tumor/*drug effects
Cytokines/*metabolism
Heat-Shock Proteins/*metabolism
Humans
Liver Neoplasms/*enzymology/*metabolism/pathology
Male
Neoplasms, Experimental/metabolism/pathology
Oxidative Stress/drug effects
Rats
Rats, Sprague-Dawley
Figure
Cited by 1 articles
-
The Risk of Gastrointestinal Cancer on Daily Intake of Low-Dose BaP in C57BL/6 for 60 Days
Zhi Zheng, Jung Kuk Park, Oh Wook Kwon, Sung Hoon Ahn, Young Joo Kwon, Linjuan Jiang, Shaohui Zhu, Byoung Hee Park
J Korean Med Sci. 2022;37(30):e235. doi: 10.3346/jkms.2022.37.e235.
Reference
-
1. The International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans: Polynuclear Aromatic Compounds, Part 1, Chemical, Environmental and Experimental Data. 1998. Lyon: The International Agency for Research on Cancer (IARC).2. Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005. 206:73–93.3. Gelboin HV. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980. 60:1107–1166.4. Lee BM, Kwack SJ, Kim HS. Age-related changes in oxidative DNA damage and benzo(a)pyrene diolepoxide-I (BPDE-I)-DNA adduct levels in human stomach. J Toxicol Environ Health A. 2005. 68:1599–1610.5. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002. 420:860–867.6. Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006. 42:745–750.7. Kahlos K, Soini Y, Pääkkö P, Säily M, Linnainmaa K, Kinnula VL. Proliferation, apoptosis, and manganese superoxide dismutase in malignant mesothelioma. Int J Cancer. 2000. 88:37–43.8. Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007. 67:3496–3499.9. Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Curr Opin Drug Discov Devel. 2006. 9:483–495.10. Marshall CJ, Vousden KH, Phillips DH. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a) pyrene diol-epoxide. Nature. 1984. 310:586–589.11. Rundle A, Tang D, Hibshoosh H, Estabrook A, Schnabel F, Cao W, Grumet S, Perera FP. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis. 2000. 21:1281–1289.12. Flowers L, Ohnishi ST, Penning TM. DNA strand scission by polycyclic aromatic hydrocarbon o-quinones: role of reactive oxygen species, Cu(II)/Cu(I) redox cycling, and o-semiquinone anion radicals. Biochemistry. 1997. 36:8640–8648.13. Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000. 13:135–160.14. Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, Che CM, Fan ST. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006. 6:1049–1057.15. Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005. 5:4581–4588.16. Sarto C, Valsecchi C, Magni F, Tremolada L, Arizzi C, Cordani N, Casellato S, Doro G, Favini P, Perego RA, Raimondo F, Ferrero S, Mocarelli P, Galli-Kienle M. Expression of heat shock protein 27 in human renal cell carcinoma. Proteomics. 2004. 4:2252–2260.17. Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000. 60:7099–7105.18. Langdon SP, Rabiasz GJ, Hirst GL, King RJ, Hawkins RA, Smyth JF, Miller WR. Expression of the heat shock protein HSP27 in human ovarian cancer. Clin Cancer Res. 1995. 1:1603–1609.19. Mehlen P, Mehlen A, Godet J, Arrigo AP. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem. 1997. 272:31657–31665.20. Lim SO, Park SG, Yoo JH, Park YM, Kim HJ, Jang KT, Cho JW, Yoo BC, Jung GH, Park CK. Expression of heat shock proteins (HSP27, HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related hepatocellular carcinomas and dysplastic nodules. World J Gastroenterol. 2005. 11:2072–2079.21. Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000. 77:396–408.22. Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996. 93:7690–7694.23. Inagaki T, Katoh K, Takiya S, Ikuta K, Kobayashi S, Suzuki M, Fukuzawa Y, Ayakawa T, Shimizu K, Tagaya T, Katoh K. Relationship between superoxide dismutase (SOD) and viral liver diseases. Gastroenterol Jpn. 1992. 27:382–389.24. Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, Tsutsui A, Okamura S, Shibata H, Ito S. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001. 21:342–349.25. Lakshmi B, Ajith TA, Jose N, Janardhanan KK. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J Ethnopharmacol. 2006. 107:297–303.26. Nishikawa T, Nakajima T, Katagishi T, Okada Y, Jo M, Kagawa K, Okanoue T, Itoh Y, Yoshikawa T. Oxidative stress may enhance the malignant potential of human hepatocellular carcinoma by telomerase activation. Liver Int. 2009. 29:846–856.27. Shin EC, Choi YH, Kim JS, Kim SJ, Park JH. Expression patterns of cytokines and chemokines genes in human hepatoma cells. Yonsei Med J. 2002. 43:657–664.28. Garcon G, Campion J, Hannothiaux MH, Boutin AC, Venembre P, Balduyck M, Haguenoer JM, Shirali P. Modification of the proteinase/anti-proteinase balance in the respiratory tract of Sprague-Dawley rats after single intratracheal instillation of benzo[A]pyrene-coated onto Fe(2)O(3) particles. J Appl Toxicol. 2000. 20:265–271.29. Lecureur V, Ferrec EL, N'Diaye M, Vee ML, Gardyn C, Gilot D, Fardel O. ERK-dependent induction of TNFalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005. 579:1904–1910.30. Chin BY, Choi ME, Burdick MD, Strieter RM, Risby TH, Choi AM. Induction of apoptosis by particulate matter: role of TNF-alpha and MAPK. Am J Physiol. 1998. 275:L942–L949.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of ganoderma incidum on mouse pulmonary adenoma induced by benzo(a)pyrene
- Induction of Heat Shock Proteins and Antioxidant Enzymes in 2,3,7,8-TCDD-Induced Hepatotoxicity in Rats
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- DNA-Protein Crosslinks Formation by Benzo[a]pyrene and the Metabolites in BALB/3T3 cells
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells