J Korean Med Sci.
2009 Oct;24(5):910-917. 10.3346/jkms.2009.24.5.910.
Weekly Paclitaxel and Trastuzumab as a First-Line Therapy in Patients with HER2-Overexpressing Metastatic Breast Cancer: Magnitude of HER2/neu Amplification as a Predictive Factor for Efficacy
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. moisa@snu.ac.kr
- 2Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1782015
- DOI: http://doi.org/10.3346/jkms.2009.24.5.910
Abstract
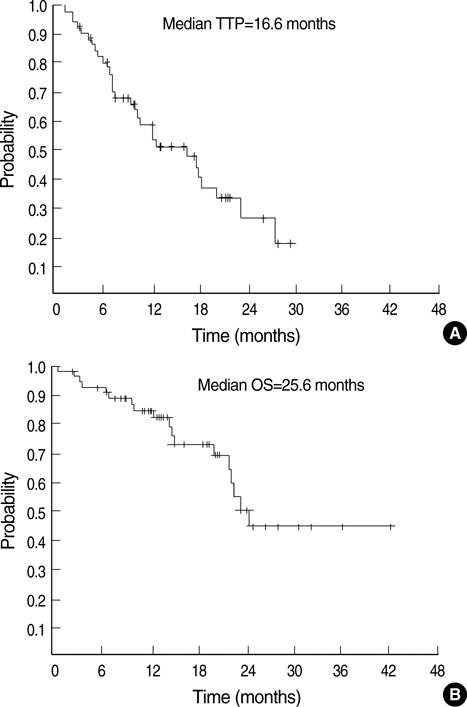
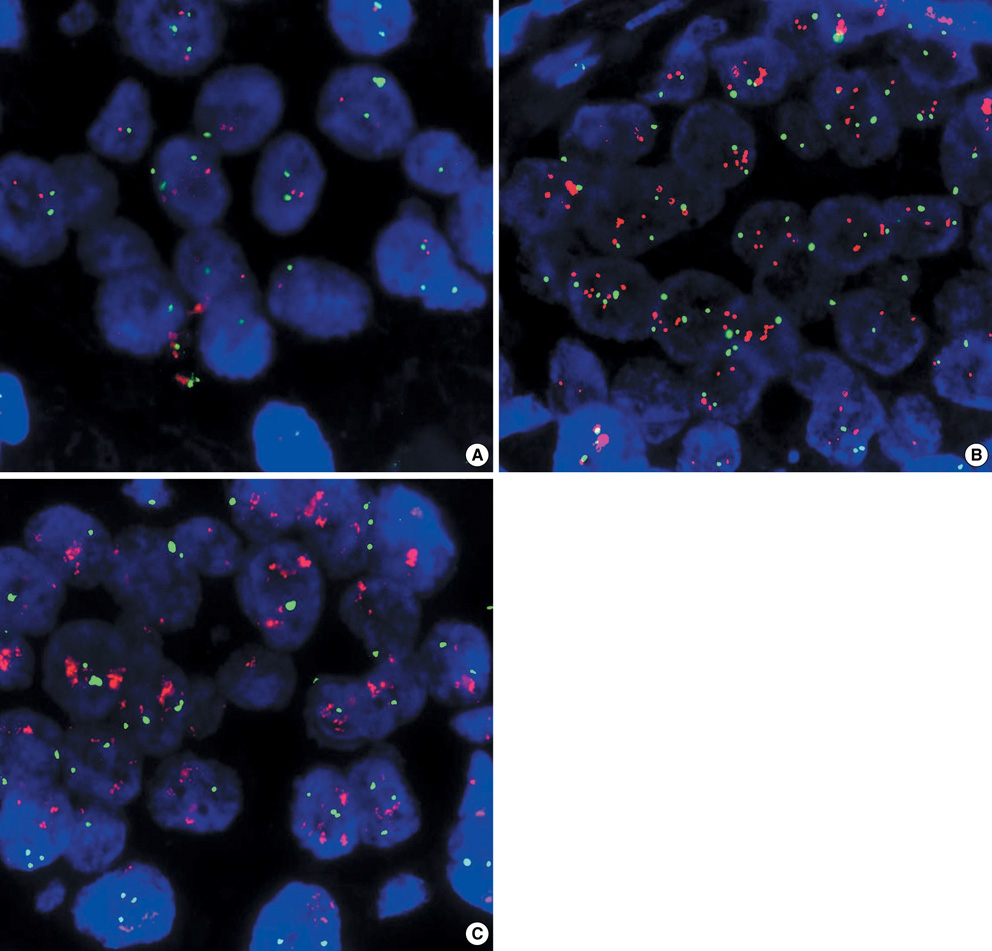
- We evaluated the efficacy and safety of weekly paclitaxel plus trastuzumab as firs-tline chemotherapy in women with HER2-overexpressing metastatic breast cancer (MBC), and we investigated the prognostic factors including magnitude of HER2/neu amplification in this population. We analyzed 54 patients with HER2-overexpressing MBC that were treated with weekly paclitaxel plus trastuzumab as first-line chemotherapy from February 2004 to December 2006. At a median follow-up of 28 months, median time to progression (TTP) was 16.6 months (95% CI, 9.4 to 23.7 months) and median overall survival was 25.6 months (95% CI, 21.8 to 27.3 months). Therapy was generally well tolerated, although three patients (5.5%) experienced reversible, symptomatic heart failure. Of the 27 patients evaluable for the HER2 FISH, patients with a HER2/CEP17 ratio of < or =4.0 had significantly shorter TTP than those with a HER2/CEP17 ratio of >4.0 (10.8 vs. 23.2 months, P=0.034). A HER2/CEP17 ratio of >4.0 was identified as significant predictive factor of TTP by multivariate analysis (P=0.032). The combination of weekly paclitaxel plus trastuzumab as first-line chemotherapy is an effective regimen in patients with HER2-FISH-positive MBC. Furthermore, the magnitude of HER2 amplification is an independent predictive factor of TTP.
MeSH Terms
-
Adult
Aged
Antibodies, Monoclonal/*administration & dosage/therapeutic use
Antineoplastic Combined Chemotherapy Protocols/*administration & dosage/therapeutic use
Breast Neoplasms/*drug therapy/mortality/pathology
Disease Progression
Drug Administration Schedule
Female
Gene Amplification
Humans
In Situ Hybridization, Fluorescence
Middle Aged
Paclitaxel/*administration & dosage/therapeutic use
Predictive Value of Tests
Receptor, erbB-2/*genetics/metabolism
Survival Analysis
Figure
Reference
-
1. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L, Wilkinson R, Qizilbash A, Ambus U, Lipa M, Weizel H, Katz A, Baida M, Mariz S, Stoik G, Dacamara P, Strongitharm D, Geddie W, McCready D. Toronto Breast Cancer Study Group. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. J Clin Oncol. 1998. 16:1340–1349.2. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007. 18:977–984.
Article3. Emens LA, Davidson NE. Trastuzumab in breast cancer. Oncology (Williston Park). 2004. 18:1117–1128.4. Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004. 96:739–749.
Article5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.
Article6. Azambuja E, Durbecq V, Rosa DD, Colozza M, Larsimont D, Piccart-Gebhart M, Cardoso F. HER-2 overexpression/amplification and its interaction with taxane-based therapy in breast cancer. Ann Oncol. 2008. 19:223–232.
Article7. Eniu A, Palmieri FM, Perez EA. Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer. Oncologist. 2005. 10:665–685.
Article8. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008. 26:1642–1649.
Article9. Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D'Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis CA. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001. 19:2587–2595.
Article10. Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, Christodoulou CH, Timotheadou E, Kalofonos CH, Kosmidis P, Adamou A, Papakostas P, Gogas H, Stathopoulos G, Razis E, Bafaloukos D, Skarlos D. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol. 2001. 12:1545–1551.11. Gori S, Colozza M, Mosconi AM, Franceschi E, Basurto C, Cherubini R, Sidoni A, Rulli A, Bisacci C, De Angelis V, Crinò L, Tonato M. Phase II study of weekly paclitaxel and trastuzumab in anthracycline-and taxane-pretreated patients with HER2-overexpressing metastatic breast cancer. Br J Cancer. 2004. 90:36–40.12. Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, Morabito A, Silingardi V, Torino F, Spada A, Zancan M, De Sio L, Caputo A, Cognetti F, Lambiase A, Amadori D. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007. 101:355–365.
Article13. Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, Untch M, Löhrs U. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001. 19:354–363.
Article14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000. 92:205–216.
Article15. Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol. 1998. 16:3353–3361.
Article16. Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001. 19:4216–4223.
Article17. Frasci G, D'Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, De Rosa V, Iodice G, Rubulotta MR, Comella G. Southern Italy Cooperative Oncology Group (SICOG). Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a sicog phase III study. Br J Cancer. 2006. 95:1005–1012.
Article18. Loesch D, Robert N, Asmar L, Gregurich MA, O'Rourke M, Dakhil S, Cox E. Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol. 2002. 20:3857–3864.
Article19. Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005. 23:7811–7819.
Article20. Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006. 24:5658–5663.
Article21. Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006. 15:219–225.
Article22. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007. 18:23–28.
Article23. Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000. 18:3651–3664.
Article24. Giuliani R, Durbecq V, Di Leo A, Paesmans M, Larsimont D, Leroy JY, Borms M, Vindevoghel A, Jerusalem G, D'Hondt V, Dirix L, Canon JL, Richard V, Cocquyt V, Majois F, Reginster M, Demol J, Kains JP, Delree P, Keppens C, Sotiriou C, Piccart MJ, Cardoso F. Phosphorylated HER-2 tyrosine kinase and Her-2/neu gene amplification as predictive factors of response to trastuzumab in patients with HER-2 overexpressing metastatic breast cancer (MBC). Eur J Cancer. 2007. 43:725–735.
Article25. Dal Lago L, Durbecq V, Desmedt C, Salgado R, Verjat T, Lespagnard L, Ma Y, Veys I, Di Leo A, Sotiriou C, Piccart M, Larsimont D. Correction for chromosome-17 is critical for the determination of true Her-2/neu gene amplification status in breast cancer. Mol Cancer Ther. 2006. 5:2572–2579.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted Therapy for Breast Cancer
- Numerical aberrations of chromosome 17 and her2/neu gene amplification, her2/neu and p 53 protein expression in breast cancer
- The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy
- HER2-Low Breast Cancer: Now and in the Future
- Personalized therapy for advanced breast cancer using molecular signatures