J Korean Med Sci.
2006 Oct;21(5):800-804. 10.3346/jkms.2006.21.5.800.
A Korean Female Patient with Thiamine-responsive Pyruvate Dehydrogenase Complex Deficiency Due to a Novel Point Mutation (Y161C)in the PDHA1 Gene
- Affiliations
-
- 1Department of Pediatrics and Research Laboratory for Human Mitochondrial Disorders, Ajou University School of Medicine, Suwon, Korea. pedkim@ajou.ac.kr
- 2Department of Pediatrics, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 1781895
- DOI: http://doi.org/10.3346/jkms.2006.21.5.800
Abstract
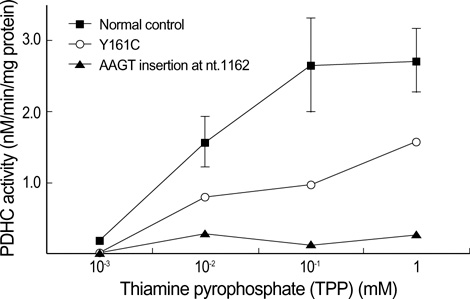
- Pyruvate dehydrogenase complex (PDHC) deficiency is mostly due to mutations in the X-linked E1alpha subunit gene (PDHA1). Some of the patients with PDHC deficiency showed clinical improvements with thiamine treatment. We report the results of biochemical and molecular analysis in a female patient with lactic acidemia. The PDHC activity was assayed at different concentrations of thiamine pyrophosphate (TPP). The PDHC activity showed null activity at low TPP concentration (1 x 10(-3) mM), but significantly increased at a high TPP concentration (1 mM). Sequencing analysis of PDHA1 gene of the patient revealed a substitution of cysteine for tyrosine at position 161 (Y161C). Thiamine treatment resulted in reduction of the patient's serum lactate concentration and dramatic clinical improvement. Biochemical, molecular, and clinical data suggest that this patient has a thiamine-responsive PDHC deficiency due to a novel mutation, Y161C. Therefore, to detect the thiamine responsiveness it is necessary to measure activities of PDHC not only at high but also at low concentration of TPP.
Keyword
MeSH Terms
Figure
Reference
-
1. Lissens W, De Meirleir L, Seneca S, Liebaers I, Brown GK, Brown RM, Ito M, Naito E, Kuroda Y, Kerr DS, Wexler ID, Patel MS, Robinson BH, Seyda A. Mutations in the X-linked pyruvate dehydrogenase (E1) α subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum Mutat. 2000. 15:209–219.
Article2. Robinson BH. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Lactic acidemia (disorders of pyruvate carboxylase, pyruvate dehydrogenase). The metabolic and molecular bases of inherited disease. 2001. 8th ed. New York: McGraw-Hill;2275–2295.3. Matsuda J, Ito M, Naito E, Yokota I, Kuroda Y. DNA diagnosis of pyruvate dehydrogenase deficiency in female patients with congenital lactic acidaemia. J Inher Metab Dis. 1995. 18:534–546.
Article4. Sheu KF, Hu CW, Utter MF. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J Clin Invest. 1981. 67:1463–1471.
Article5. Naito E, Ito M, Yokota I, Saijo T, Matsuda J, Ogawa Y, Kitamura S, Takada E, Horii Y, Kuroda Y. Thiamine-responsive pyruvate dehydrogenase deficiency in two patients caused by a point mutation (F205L and L216F) within the thiamine pyrophosphate binding region. Biochim Biophys Acta. 2002. 1588:79–84.
Article6. Naito E, Ito M, Takeda E, Yokota I, Yoshijima S, Kuroda Y. Molecular analysis of abnormal pyruvate dehydrogenase in a patient with thiamine-responsive congenital lactic academia. Pediatr Res. 1994. 36:340–346.7. Di Rocco M, Lamba LD, Minniti G, Caruso U, Naito E. Outcome of thiamine treatment in a child with Leigh disease due to thimanine-responsive pyruvate dehydrogenase deficiency. Eur J Paediatr Neurol. 2000. 4:115–117.8. Saijo T, Naito E, Ito M, Yokota I, Matsuda J, Kuroda Y. Stable restoration of pyruvate dehydrogenase complex in E1-defective human lymphoblastoid cells: Evidence that three C-terminal amino acids of E1α are essential for the structural integrity of heterotetrameric E1. Biochem Biophys Res Commun. 1996. 228:446–451.
Article9. Benelli C, Fouque F, Redonnet-Vernhet I, Malgat M, Fontan D, Marsac C, Dey R. A novel Y243S mutation in the pyruvate dehydrogenase E1 alpha gene subunit: Correlation with thiamine pyrophosphate interaction. J Inher Metab Dis. 2002. 25:325–327.
Article10. Kitamori N, Itokawa Y. Pharmacokinetics of thiamine after oral administration of thiamine tetrahydrofurfuryl disulfide to humans. J Nutr Sci Vitaminol. 1993. 39:465–472.11. Robinson BH, Chun K. The relationships between transketolase, yeast pyruvate decarboxylase and pyruvate dehydrogenase of the pyruvate dehydrogenase complex. FEBS Lett. 1993. 328:99–102.
Article12. Naito E, Ito M, Yokota I, Saijo T, Chen S, Maehara M, Kuroda Y. Concomitant administration of sodium dichloroacetate and thiamine in West syndrome caused by thiamine-responsive pyruvate dehydrogenase complex deficiency. J Neurol Sci. 1999. 171:56–59.
Article13. Naito E, Ito M, Yokota I, Saijo T, Matsuda J, Osaka H, Kimura S, Kuroda Y. Biochemical and molecular analysis of an X-linked case of Leigh syndrome associated with thiamine-responsive pyruvate dehydrogenase deficiency. J Inherit Metab Dis. 1997. 20:539–548.14. Marsac C, Benelli C, Desguerre I, Diry M, Fouque F, De Meirleir L, Ponsot G, Seneca S, Poggi F, Saudubray JM, Zabot MT, Fontan D, Lissens W. Biochemical and genetic studies of four patients with pyruvate dehydrogenase E1α deficiency. Hum Genet. 1997. 99:785–792.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases
- Anesthetic experience in a pediatric patient with pyruvate dehydrogenase complex (PDHC) deficiency: A case report
- A case of lactic acidosis caused by thiamine deficiency
- The Role of Pyruvate Dehydrogenase Kinase in Diabetes and Obesity
- Perioperative considerations of pyruvate dehydrogenase complex deficiency: a case report of two consecutive anesthesia