Korean J Lab Med.
2008 Feb;28(1):53-63. 10.3343/kjlm.2008.28.1.53.
Protein Profile Changes in Platelet Concentrates According to Storage and Leukoreduction- Analysis Using Proteomics Technology
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Kangwon National University, Chuncheon, Korea. eqcho1ku@korea.ac.kr
- 2Department of Laboratory Medicine, College of Medicine, Korea University, Seoul, Korea.
- KMID: 1781559
- DOI: http://doi.org/10.3343/kjlm.2008.28.1.53
Abstract
-
BACKGROUND: Knowing how the protein profile of platelet products changes with storage or leukoreduction may give us greater insight into cell physiology and the cause of transfusion reactions other than cytokines and chemokines.
METHODS
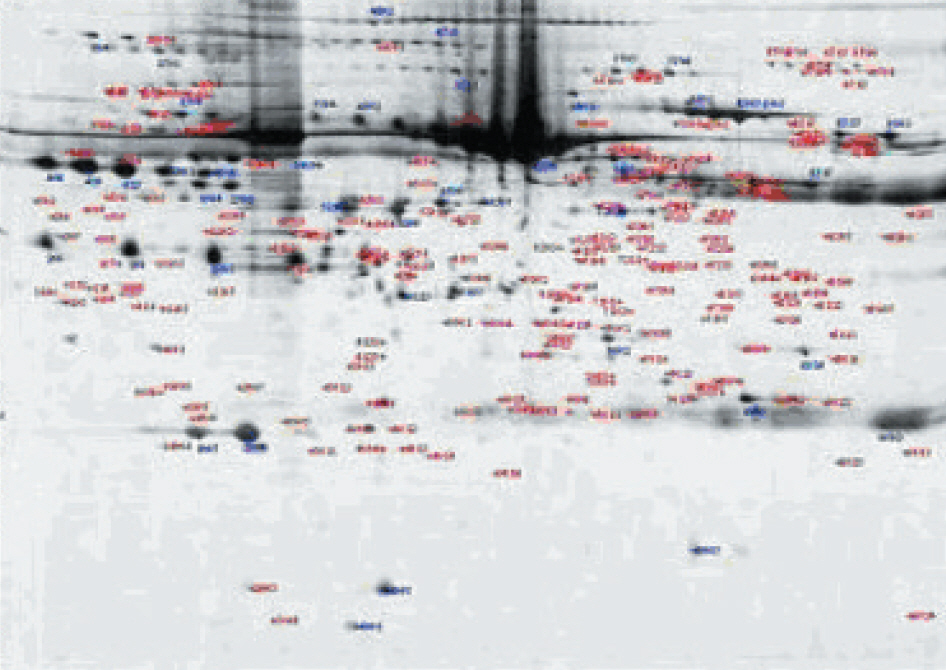
We filtered four packs of platelet concentrates (PC) within 24 hr of blood collection and after 120 hrs of storage. Four aliquots of each supernatant in PC were obtained: pre-storage+prefiltration, pre-storage+post-filtration, post-storage+pre-filtration and post-storage+post-filtration. Routine chemistry tests and a two-dimensional electrophoresis (2-DE) were performed. The stained images were analyzed and the significant spots were identified using a peptide mass finger printing (PMF) with matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis after trypsin digestion.
RESULTS
The protein spots increased with storage and decreased after filtration (P<0.05, prestorage+post-filtration). The spot density of various proteins, including macrophage inflammatory protein-2 alpha, megakaryocyte colony stimulating factor and interleukin-22 changed with storage and leukoreduction.
CONCLUSIONS
The database of identified protein spots and their changes produced in this study is a useful basic tool for future studies on the mechanism of transfusion reactions. Further studies should validate the significance of each protein spot.
Keyword
MeSH Terms
Figure
Reference
-
1.Heddle NM. Febrile nonhemolytic transfusion reactions to platelets. Curr Opin Hematol. 1995. 2:478–83.
Article2.Seghatchian J., Krailadsiri P., Dilger P., Thorpe R., Wadhwa M. Cytokines as quality indicators of leucoreduced red cell concentrates. Transfus Apher Sci. 2002. 26:43–6.
Article3.Boehlen F., Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001. 11:403–17.
Article4.Muylle L., Peetermans ME. Effect of prestorage leukocyte removal on the cytokine levels in stored platelet concentrates. Vox Sang. 1994. 66:14–7.
Article5.Yazer MH., Podlosky L., Clarke G., Nahirniak SM. The effect of pre-storage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004. 44:10–5.
Article6.Paglino JC., Pomper GJ., Fisch GS., Champion MH., Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004. 44:16–24.
Article7.Kluter H., Bubel S., Kirchner H., Wilhelm D. Febrile and allergic transfusion reactions after the transfusion of white cell-poor platelet preparations. Transfusion. 1999. 39:1179–84.8.Miletic VD., Popovic O. Complement activation in stored platelet concentrates. Transfusion. 1993. 33:150–4.
Article9.Silliman CC., Johnson CA., Clay KL., Thurman GW., Ambruso DR. Compounds biologically similar to platelet activating factor are present in stored blood components. Lipids. 1993. 28:415–8.
Article10.Heddle NM., Klama L., Singer J., Richards C., Fedak P., Walker I, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994. 331:625–8.
Article11.Muylle L., Joos M., Wouters E., De Bock R., Peetermans ME. Increased tumor necrosis factor alpha (TNF alpha), interleukin 1, and inter-leukin 6 (IL-6) levels in the plasma of stored platelet concentrates: relationship between TNF alpha and IL-6 levels and febrile transfusion reactions. Transfusion. 1993. 33:195–9.
Article12.Seghatchian J. The platelet storage lesion: A comparative analysis of six leukoreduction processes in terms of biocompatability, microve-siculation, retention of prions, and generation/removal of biological response modifiers. Transfus Apher Sci. 2005. 15:[Epub ahead of print].13.Zhou M., Conrads TP., Veenstra TD. Proteomics approaches to bio-marker detection. Brief Funct Genomic Proteomic. 2005. 4:69–75.14.Omenn GS., States DJ., Adamski M., Blackwell TW., Menon R., Hermjakob H, et al. Overview of the HUPO Plasma Proteome Project: results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics. 2005. 5:3226–45.
Article15.Reddy KS., Perrotta PL. Proteomics in transfusion medicine. Transfusion. 2004. 44:601–4.
Article16.Sarkodee-Adoo CB., Kendall JM., Sridhara R., Lee EJ., Schiffer CA. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion. 1998. 38:229–35.
Article17.Omenn GS. The Human Proteome Organization Plasma Proteome Project pilot phase: reference specimens, technology platform comparisons, and standardized data submissions and analyses. Proteomics. 2004. 4:1235–40.
Article18.O'Neill EE., Brock CJ., von Kriegsheim AF., Pearce AC., Dwek RA., Watson SP, et al. Towards complete analysis of the platelet proteome. Proteomics. 2002. 2:288–305.19.Garcia A., Zitzmann N., Watson SP. Analyzing the platelet proteome. Semin Thromb Hemost. 2004. 30:485–9.
Article20.Kakhniashvili DG., Bulla LA Jr., Goodman SR. The human erythrocyte proteome: analysis by ion trap mass spectrometry. Mol Cell Proteomics. 2004. 3:501–9.21.Anderson NL., Polanski M., Pieper R., Gatlin T., Tirumalai RS., Conrads TP, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004. 3:311–26.22.Anniss AM., Glenister KM., Killian JJ., Sparrow RL. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005. 45:1426–33.
Article23.Thiele T., Steil L., Gebhard S., Scharf C., Hammer E., Brigulla M, et al. Profiling of alterations in platelet proteins during storage of platelet concentrates. Transfusion. 2007. 47:1221–33.
Article24.Ledent E., Berlin G. Factors influencing white cell removal from red cell concentrates by filtration. Transfusion. 1996. 36:714–8.
Article25.Aye MT., Palmer DS., Giulivi A., Hashemi S. Effect of filtration of platelet concentrates on the accumulation of cytokines and platelet release factors. Transfusion. 1995. 35:117–24.26.Wolpe SD., Sherry B., Juers D., Davatelis G., Yurt RW., Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989. 86:612–6.
Article27.King AG., Johanson K., Frey CL., DeMarsh PL., White JR., McDevitt P, et al. Identification of unique truncated KC/GRO beta chemokines with potent hematopoietic and anti-infective activities. J Immunol. 2000. 164:3774–82.28.Driscoll KE. TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicol Lett. 2000. 112-113:177–83.29.Fishley B., Alexander WS. Thrombopoietin signalling in physiology and disease. Growth Factors. 2004. 22:151–5.
Article30.Xie MH., Aggarwal S., Ho WH., Foster J., Zhang Z., Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000. 275:31335–9.
Article31.Boniface K., Bernard FX., Garcia M., Gurney AL., Lecron JC., Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005. 174:3695–702.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Status of Use of Leukoreduced Blood Products in Korean Hospitals
- Effect of Interrupted Agitation and Removal of Leukocyte on Platelet Quality during the Storage of Platelet Concentrates
- Evaluation of a domestic second generation platelet storage container
- Evaluation of Platelet Concentrates Stored in the Second Generation Platelet Storage Containers
- Filtration Effects of Leukocyte Removal Filter for Platelet Concentrates