Yonsei Med J.
2012 Jan;53(1):204-212. 10.3349/ymj.2012.53.1.204.
Ionic Mechanisms of Desflurane on Prolongation of Action Potential Duration in Rat Ventricular Myocytes
- Affiliations
-
- 1Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Life Science, College of Natural Sciences, Ewha Woman's University, Seoul, Korea.
- 3Department of Physiology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. wkp7ark@yuhs.ac
- KMID: 1779708
- DOI: http://doi.org/10.3349/ymj.2012.53.1.204
Abstract
- PURPOSE
Despite the fact that desflurane prolongs the QTC interval in humans, little is known about the mechanisms that underlie these actions. We investigated the effects of desflurane on action potential (AP) duration and underlying electrophysiological mechanisms in rat ventricular myocytes.
MATERIALS AND METHODS
Rat ventricular myocytes were enzymatically isolated and studied at room temperature. AP was measured using a current clamp technique. The effects of 6% (0.78 mM) and 12% (1.23 mM) desflurane on transient outward K+ current (I(to)), sustained outward current (I(sus)), inward rectifier K+ current (I(KI)), and L-type Ca2+ current were determined using a whole cell voltage clamp.
RESULTS
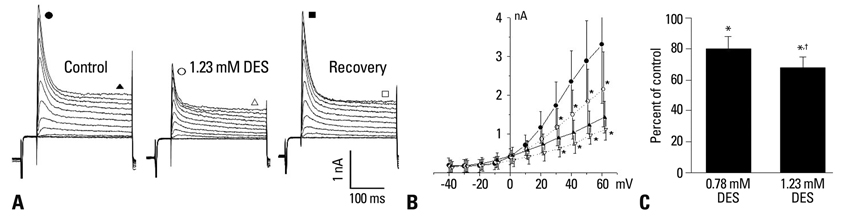
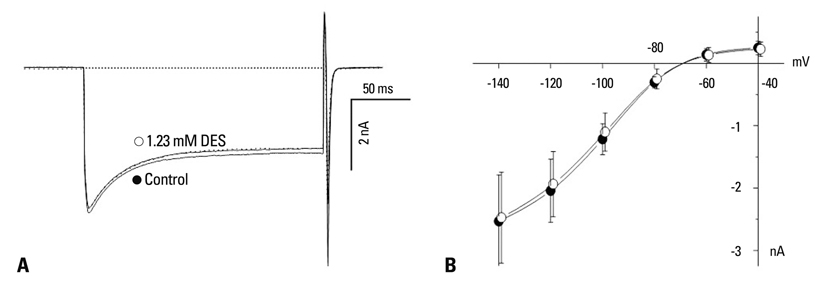
Desflurane prolonged AP duration, while the amplitude and resting membrane potential remained unchanged. Desflurane at 0.78 mM and 1.23 mM significantly reduced the peak I(to) by 20+/-8% and 32+/-7%, respectively, at +60 mV. Desflurane (1.23 mM) shifted the steady-state inactivation curve in a hyperpolarizing direction and accelerated inactivation of the current. While desflurane (1.23 mM) had no effects on I(sus) and I(KI), it reduced the L-type Ca2+ current by 40+/-6% (p<0.05).
CONCLUSION
Clinically relevant concentrations of desflurane appear to prolong AP duration by suppressing Ito in rat ventricular myocytes.
MeSH Terms
-
Action Potentials/*drug effects
Anesthetics, Inhalation/*pharmacology
Animals
Calcium Channels, L-Type/physiology
Heart Conduction System/drug effects/physiology
Heart Ventricles/drug effects
Isoflurane/*analogs & derivatives/pharmacology
Myocardial Contraction/*drug effects/physiology
Myocytes, Cardiac/*drug effects/physiology
Patch-Clamp Techniques
Potassium Channels/physiology
Rats
Rats, Sprague-Dawley
Figure
Reference
-
1. Yildirim H, Adanir T, Atay A, Katirciolu K, Savaci S. The effects of sevoflurane, isoflurane and desflurane on QT interval of the ECG. Eur J Anaesthesiol. 2004. 21:566–570.
Article2. Silay E, Kati I, Tekin M, Guler N, Huseyinoglu UA, Coskuner I, et al. Comparison of the effects of desflurane and sevoflurane on the QTc interval and QT dispersion. Acta Cardiol. 2005. 60:459–464.
Article3. Owczuk R, Wujtewicz MA, Sawicka W, Lasek J, Wujtewicz M. The Influence of desflurane on QTc interval. Anesth Analg. 2005. 101:419–422.
Article4. Aypar E, Karagoz AH, Ozer S, Celiker A, Ocal T. The effects of sevoflurane and desflurane anesthesia on QTc interval and cardiac rhythm in children. Paediatr Anaesth. 2007. 17:563–567.
Article5. Park WK, Kim MH, Ahn DS, Chae JE, Jee YS, Chung N, et al. Myocardial depressant effects of desflurane: mechanical and electrophysiologic actions in vitro. Anesthesiology. 2007. 106:956–966.6. Carmeliet E. Mechanisms and control of repolarization. Eur Heart J. 1993. 14:Suppl H. 3–13.
Article7. Coraboeuf E. Ionic basis of electrical activity in cardiac tissues. Am J Physiol. 1978. 234:H101–H116.
Article8. Rees S, Curtis MJ. Which cardiac potassium channel subtype is the preferable target for suppression of ventricular arrhythmias? Pharmacol Ther. 1996. 69:199–217.
Article9. Wettwer E, Amos G, Gath J, Zerkowski HR, Reidemeister JC, Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovasc Res. 1993. 27:1662–1669.
Article10. Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993. 73:386–394.
Article11. Nánási PP, Varró A, Lathrop DA. Ionic currents in ventricular myocytes isolated from the heart of a patient with idiopathic cardiomyopathy. Cardioscience. 1992. 3:85–89.12. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.
Article13. Castle NA. Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J Pharmacol Exp Ther. 1990. 255:1038–1046.14. Slawsky MT, Castle NA. K+ channel blocking actions of flecainide compared with those of propafenone and quinidine in adult rat ventricular myocytes. J Pharmacol Exp Ther. 1994. 269:66–74.15. He J, Kargacin ME, Kargacin GJ, Ward CA. Tamoxifen inhibits Na+ and K+ currents in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2003. 285:H661–H668.16. Apkon M, Nerbonne JM. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991. 97:973–1011.
Article17. Hönemann CW, Washington J, Hönemann MC, Nietgen GW, Durieux ME. Partition coefficients of volatile anesthetics in aqueous electrolyte solutions at various temperatures. Anesthesiology. 1998. 89:1032–1035.
Article18. Hume JR, Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983. 81:153–194.
Article19. Hume JR, Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985. 368:525–544.
Article20. Josephson IR, Sanchez-Chapula J, Brown AM. Early outward current in rat single ventricular cells. Circ Res. 1984. 54:157–162.
Article21. Litovsky SH, Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988. 62:116–126.
Article22. Giles W, Shimoni Y. Comparison of sodium-calcium exchanger and transient inward currents in single cells from rabbit ventricle. J Physiol. 1989. 417:465–481.
Article23. Coraboeuf E, Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982. 392:352–359.
Article24. Escande D, Coulombe A, Faivre JF, Deroubaix E, Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987. 252:H142–H148.
Article25. Dukes ID, Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol. 1991. 435:395–420.
Article26. Dukes ID, Cleemann L, Morad M. Tedisamil blocks the transient and delayed rectifier K+ currents in mammalian cardiac and glial cells. J Pharmacol Exp Ther. 1990. 254:560–569.27. Walker MJ, Beatch GN. Electrically induced arrhythmias in the rat. Proc West Pharmacol Soc. 1988. 31:167–170.28. Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circ Res. 1996. 78:1–7.
Article29. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. The Sicilian gambit. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Circulation. 1991. 84:1831–1851.30. Gueugniaud PY, Hanouz JL, Vivien B, Lecarpentier Y, Coriat P, Riou B. Effects of desflurane in rat myocardium: comparison with isoflurane and halothane. Anesthesiology. 1997. 87:599–609.31. Chae JE, Ahn DS, Kim MH, Lynch C 3rd, Park WK. Electrophysiologic mechanism underlying action potential prolongation by sevoflurane in rat ventricular myocytes. Anesthesiology. 2007. 107:67–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Electrophysiologic Effect of Desflurane on the Prolongation of Action Potential Duration in Ventricular Myocytes
- Electrophysiologic Mechanisms of Sevoflurane on Prolongation of the QT Interval: K+ Currents in Rat Ventricular Myocytes
- Effects of ryanodine on the intracellular Na+ activity and tension and action potentials of rat and guinea pig cardiac ventricular muscles
- Effect of Cisapride on ATP-sensitive K Channel of Ventricular Cell
- Direct Myocardial Depressant Effect of Desflurane: Mechanical and Electrophysiological Actions in Vitro