Yonsei Med J.
2006 Aug;47(4):505-512. 10.3349/ymj.2006.47.4.505.
Evaluating the Allergic Risk of Genetically Modified Soybean
- Affiliations
-
- 1Department of Allergy and Rheumatology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Food science and Technology, Seoul National University, Seoul, Korea.
- 3College of Agriculture, Chonbuk National University, Chonju, Korea.
- KMID: 1779500
- DOI: http://doi.org/10.3349/ymj.2006.47.4.505
Abstract
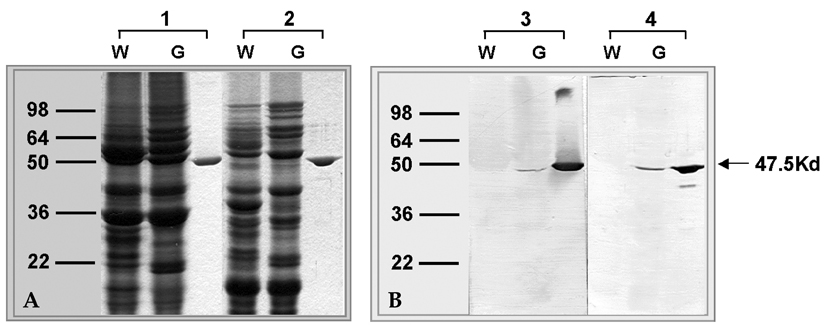
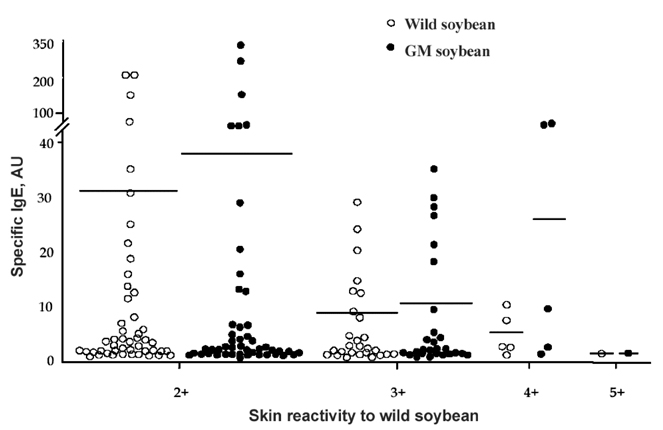
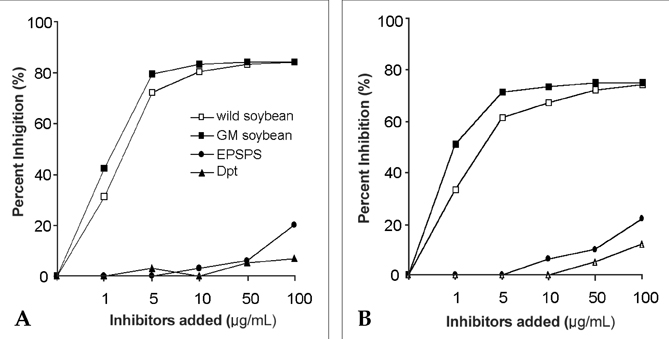
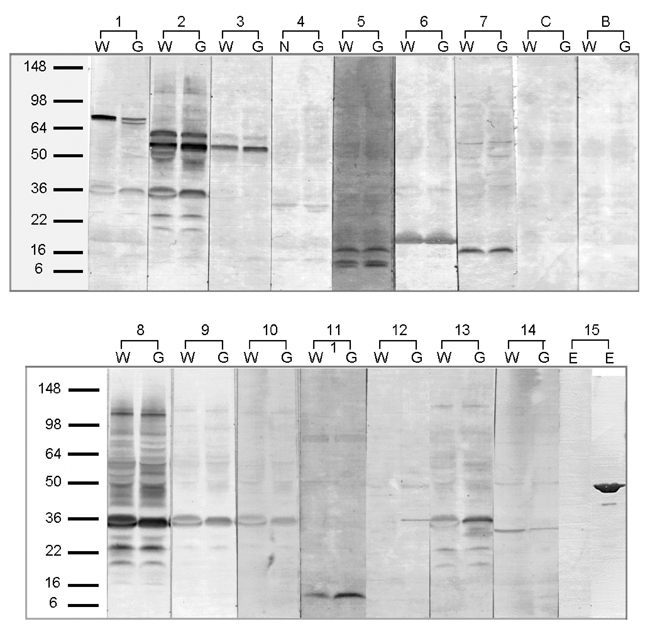
- Genetically modified (GM) soybean (carrying the EPSPS transgene) is the most common GM food in Korea. In order to assess whether genetic modification increases the allergenic risk of soybeans, the allergenicity and IgE-reactive components of wild-type and GM soybean extracts were compared in allergic adults who had been sensitized to soybeans. We enrolled 1,716 adult allergy patients and 40 healthy, non-atopic controls. Skin prick tests and IgE enzyme linked immunosorbent assays (ELISAs) were performed using wild-type and GM soybean extracts, along with other common inhaled allergens. The specificities of serum IgE antibodies from allergic patients and the identities of the IgE-reactive components of the soybean extracts were compared using ELISA inhibition testing, 2-dimensional gel electrophoresis, and IgE immunoblotting. To evaluate the effects of digestive enzymes and heat treatment, the soybean extracts were heated or pre- incubated with or without simulated gastric and intestinal fluids. The IgE sensitization rates to wild-type and GM soybeans were identical (3.8% of allergic adults), and circulating IgE antibodies specific for the two extracts were comparable. The results of the ELISA inhibition test, SDS-PAGE, and IgE immunoblotting showed a similar composition of IgE-binding components within the wild-type and GM extracts, which was confirmed using two-dimensional gel electrophoresis, IgE immunoblotting, and amino acid sequencing. None of the subjects had a positive response to purified EPSPS protein in the skin prick test, ELISA, or IgE immunoblot analysis. These findings suggest that the IgE sensitization rate to GM soybean extracts is identical to that of wild-type soybean extracts in adult allergy patients. In addition, based on both in vivo and in vitro methods, the allergenicity of wild type and GM soybean extracts was identical.
Keyword
MeSH Terms
-
Soybeans/*immunology
Skin Tests
Protein Structure, Tertiary
*Plants, Genetically Modified
Middle Aged
Immunoglobulin E/blood/chemistry
Immunoblotting
Humans
Food Hypersensitivity/etiology/*immunology
Food/*adverse effects
Enzyme-Linked Immunosorbent Assay
Electrophoresis, Gel, Two-Dimensional
*Crops, Agricultural
Allergens/*immunology
Adult
Adolescent
Figure
Cited by 2 articles
-
Identification of Dioscorea Batatas (Sanyak) Allergen as an Inhalant and Oral Allergen
Gyu-Young Hur, Han-Jung Park, Hyoun-Ah Kim, Young-Min Ye, Hae-Sim Park
J Korean Med Sci. 2008;23(1):72-76. doi: 10.3346/jkms.2008.23.1.72.Comparative Analysis of Immunoreactivity between Individual Serum and Pooled Serum in Serum Screening
Jinyoung Lee, Jeong Ok Lee, Jihyun Kim, Youngshin Han, Kangmo Ahn
Pediatr Allergy Respir Dis. 2012;22(4):390-396. doi: 10.7581/pard.2012.22.4.390.
Reference
-
1. Jung JA, Nam SY, Han YS, Park YM, Lee JS, Jeon KH, et al. The sensitization rates to egg, milk, soy bean in children with atopic dermatitis and acute urticaria. J Asthma Allergy Clin Immunol. 2001. 21:610–617.2. Kim SH, Kang HR, Kim KM, Kim TB, Kim SS, Chang YS, et al. The sensitization rates of food allergens in a Korean population: a multi-center study. J Asthma Allergy Clin Immunol. 2003. 23:502–504.3. Lack G, Chapman M, Kalsheker N, King V, Robinson C, Venables K. BSACI working party. Report on the potential allergenicity of genetically modified organisms and their products. Clin Exp Allergy. 2002. 32:1131–1143.4. Sutton SA, Assa'ad AH, Steinmetz C, Rothenberg ME. A negative, double-blind, placebo-controlled challenge to genetically modified corn. J Allergy Clin Immunol. 2003. 112:1011–1012.5. Batista R, Nunes B, Carmo M, Cardoso C, Jose HS, de Almeida AB, et al. Lack of detectable allergenicity of transgenic maize and soya samples. J Allergy Clin Immunol. 2005. 116:403–410.6. Kim YK, Park HS, Kim HA, Lee MH, Choi JH, Kim SS, et al. Two-spotted spider mite allergy: immunoglobulin E sensitization and characterization of allergenic components. Ann Allergy Asthma Immunol. 2002. 89:517–522.7. Evaluation of allergenicity of genetically modified foods. Report of a Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology. 2001. 1–19.8. Altschul SF, Madden TL, Schaffer AA, Zang J, Zang Z, Miller W, et al. A new generation of protein database search programs. Nucleic Acids Res. 1997. 25:3389–3402.9. Kalinski A, Weisemann JM, Matthews BF, Herman EM. Molecular cloning of a protein associated with soybean seed oil bodies that is similar to thiol proteases of the papain family. J Biol Chem. 1990. 265:13843–13848.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Allergenicity to Genetically Modified Organic Foods
- Assessment of Allergenicity in Genetically Modified Herbicide-Resistant Foods Using the Serum Screening Test
- Comparison of Genetically Modified Soybean and Wild Soybean in Physicochemical Aspects
- Comparison of regulatory framework of clinical trial with genetically modified organism-containing vaccines in the Europe, Australia, and Switzerland
- Biohazard surveillance of allergic contact dermatitis in genetically-modified Zoysia grasses using patch testing