Korean J Radiol.
2009 Jun;10(3):285-293. 10.3348/kjr.2009.10.3.285.
Multislice CT Angiography of Fenestrated Endovascular Stent Grafting for Treating Abdominal Aortic Aneurysms: a Pictorial Review of the 2D/3D Visualizations
- Affiliations
-
- 1Discipline of Medical Imaging, Department of Imaging and Applied Physics, Curtin University of Technology, Perth, Western Australia, Australia. z.sun@curtin.edu.au
- 2Department of Vascular Surgery, Royal Perth Hospital, School of Medicine and Pathology, University of Western Australia, Western Australia, Australia.
- 3Cook R & D, Perth, Western Australia, Australia.
- 4School of Public Health, Curtin University of Technology, Perth, Western Australia, Australia.
- KMID: 1779456
- DOI: http://doi.org/10.3348/kjr.2009.10.3.285
Abstract
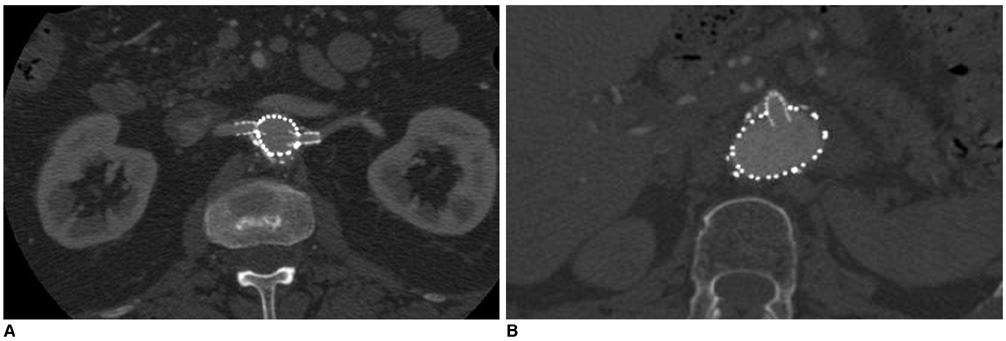
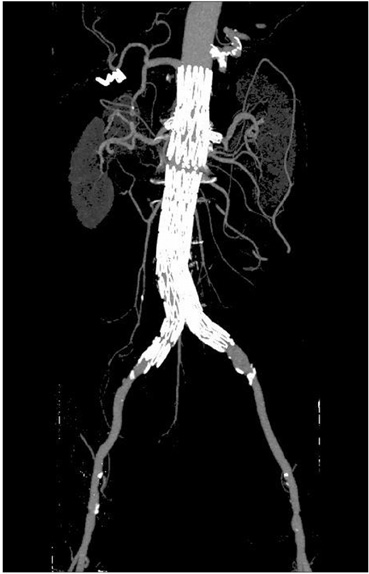
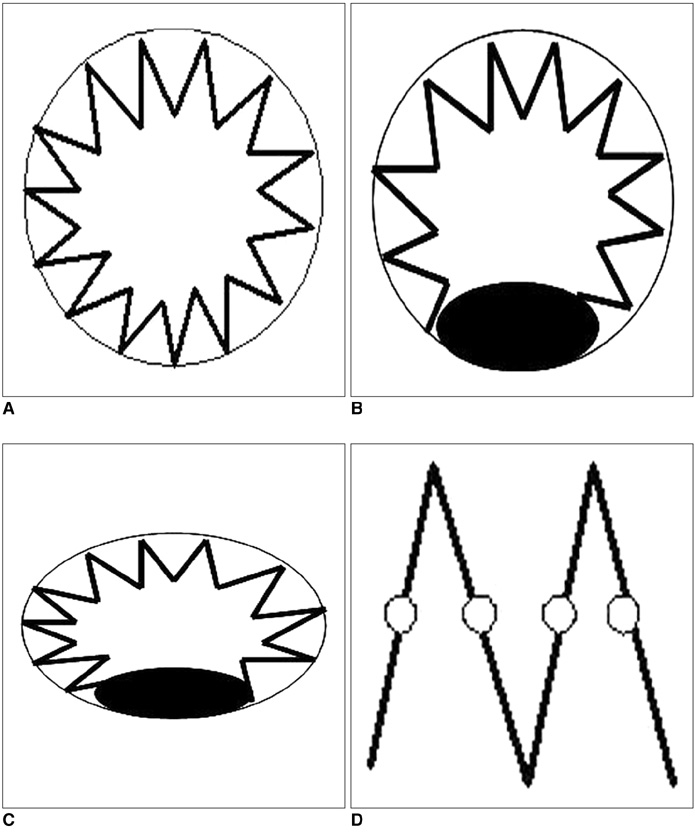
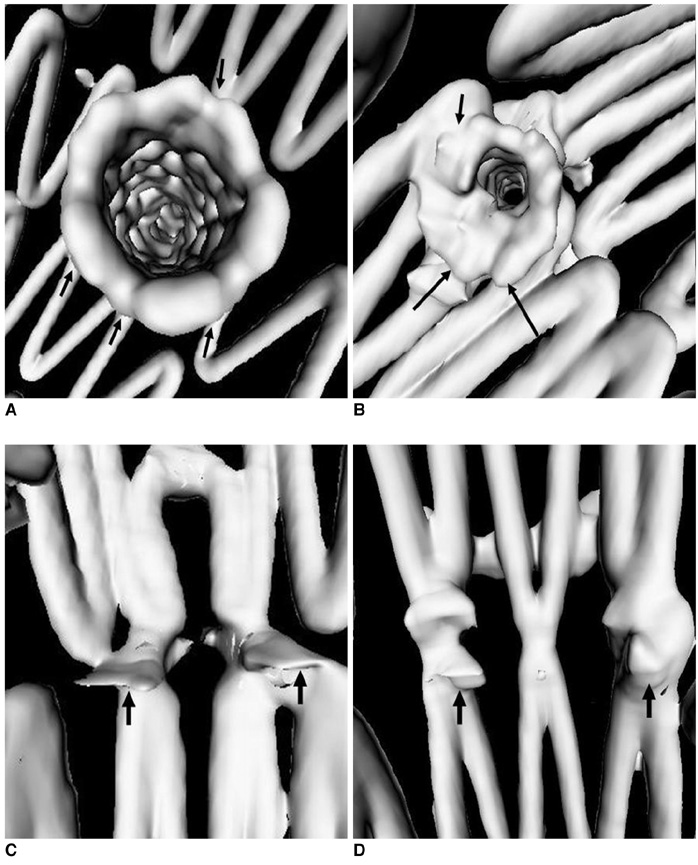
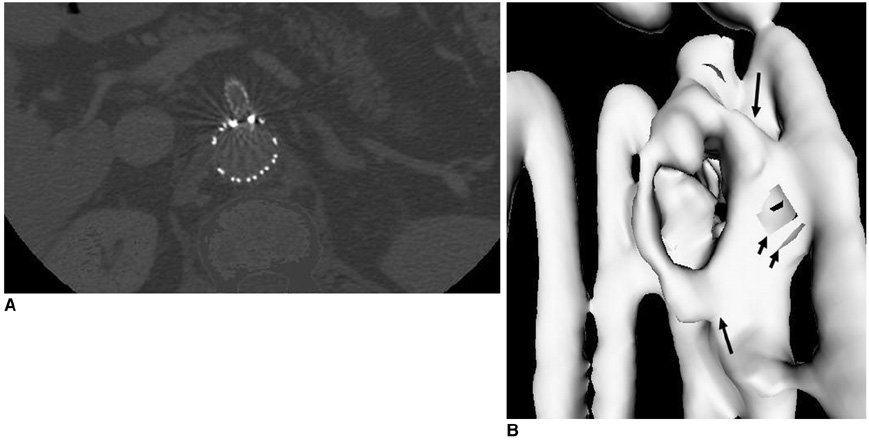
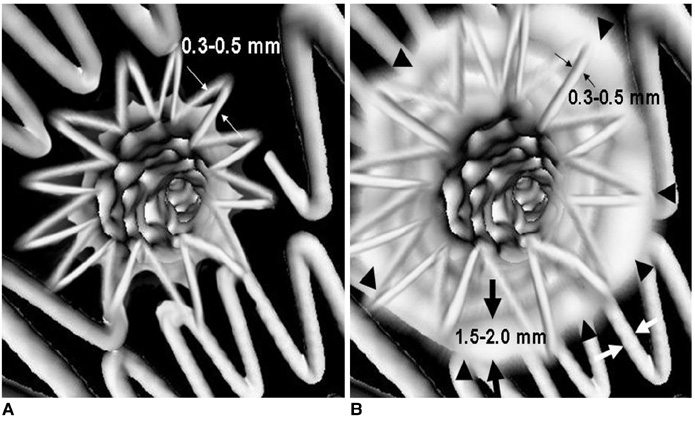
- Fenestrated endovascular repair of an abdominal aortic aneurysm has been developed to treat patients with a short or complicated aneurysm neck. Fenestration involves creating an opening in the graft fabric to accommodate the orifice of the vessel that is targeted for preservation. Fixation of the fenestration to the renal arteries and the other visceral arteries can be done by implanting bare or covered stents across the graft-artery ostia interfaces so that a portion of the stent protrudes into the aortic lumen. Accurate alignment of the targeted vessels in a longitudinal aspect is hard to achieve during stent deployment because rotation of the stent graft may take place during delivery from the sheath. Understanding the 3D relationship of the aortic branches and the fenestrated vessel stents following fenestration will aid endovascular specialists to evaluate how the stent graft is situated within the aorta after placement of fenestrations. The aim of this article is to provide the 2D and 3D imaging appearances of the fenestrated endovascular grafts that were implanted in a group of patients with abdominal aortic aneurysms, based on the multislice CT angiography. The potential applications of each visualization technique were explored and compared with the 2D axial images.
MeSH Terms
-
Aged
Aged, 80 and over
Aorta, Abdominal/radiography
Aortic Aneurysm, Abdominal/*radiography/*surgery
*Blood Vessel Prosthesis
Contrast Media/administration & dosage
Female
Humans
Image Processing, Computer-Assisted/methods
Imaging, Three-Dimensional/*methods
Iohexol/administration & dosage/analogs & derivatives
Male
Middle Aged
Prosthesis Design
Radiographic Image Enhancement/methods
*Stents
Tomography, X-Ray Computed/*methods
Figure
Reference
-
1. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991. 5:491–499.2. Buth J, van Marrewijk CJ, Harris PL, Hop WC, Riambau V, Laheij RJ. EUROSTAR Collaborators. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: a report on the EUROSTAR experience. J Vasc Surg. 2002. 35:211–221.3. Lobato AC, Quick RC, Vaughn PL, Rodriguez-Lopez J, Douglas M, Diethrich EB. Transrenal fixation of aortic endografts: intermediate follow-up of a single e-center experience. J Endovasc Ther. 2000. 7:273–278.4. Bove PG, Long GW, Zelenock GB, Bendick PJ, Khoury MD, Burr MO, et al. Transrenal fixation of aortic stent-grafts for the treatment of infrarenal aortic aneurysmal disease. J Vasc Surg. 2000. 32:697–703.5. Browne TF, Hartley D, Purchas S, Rosenberg M, Van Schie G, Lawrence-Brown M. A fenestrated covered suprarenal aortic stent. Eur J Vasc Endovasc Surg. 1999. 18:445–449.6. Stanley BM, Semmens JB, Lawrence-Brown MM, Goodman MA, Hartley DE. Fenestration in endovascular grafts for aortic aneurysm repair: new horizons for preserving blood flow in branch vessels. J Endovasc Ther. 2001. 8:16–24.7. Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther. 2001. 8:3–15.8. Sun Z, Winder RJ, Kelly BE, Ellis PK, Kennedy PT, Hirst DG. Diagnostic value of CT virtual intravascular endoscopy in aortic stent-grafting. J Endovasc Ther. 2004. 11:13–25.9. Sun Z. 3D multislice CT angiography in post-aortic stent grafting: a pictorial essay. Korean J Radiol. 2006. 7:205–211.10. Sun Z. Three-dimensional visualization of suprarenal aortic tent-grafts: evaluation of migration in midterm follow-up. J Endovasc Ther. 2006. 13:85–93.11. Sun Z, Winder RJ, Kelly BE, Ellis PK, Hirst DG. CT virtual intravascular endoscopy of abdominal aortic aneurysms treated with suprarenal endovascular stent grafting. Abdom Imaging. 2003. 28:580–587.12. Sun Z. Transrenal fixation of aortic stent-grafts: current status and future directions. J Endovasc Ther. 2004. 11:539–549.13. Sun Z. Helical CT angiography of abdominal aortic aneurysms treated with suprarenal stent grafting. Cardiovasc Intervent Radiol. 2003. 26:290–295.14. Sun Z, Allen YB, Nadkarni S, Knight R, Hartley D, Lawrence-Brown MM. CT virtual intravascular endoscopy in the visualization of fenestrated stent-grafts. J Endovasc Ther. 2008. 15:42–51.15. Sun Z, Mwipatayi BP, Semmens JB, Lawrence-Brown MM. Short to midterm outcomes of fenestrated endovascular grafts in the treatment of abdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2006. 13:747–753.16. Semmens JB, Lawrence-Brown MM, Hartley DE, Allen YB, Green R, Nadkarni S. Outcomes of fenestrated endografts in the treatment of abdominal aortic aneurysm in Western Australia (1997-2004). J Endovasc Ther. 2006. 13:320–329.17. Sun Z, Allen YB, Mwipatayi BP, Hartley DE, Lawrence-Brown MM. Multislice CT angiography in the follow-up of fenestrated endovascular grafts: effect of slice thickness on 2D and 3D visualization of the fenestrated stents. J Endovasc Ther. 2008. 15:417–426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 3D Multislice CT Angiography in Post-Aortic Stent Grafting: A Pictorial Essay
- Endovascular Repair of Thoracic Aortic Aneurysm Using a Custom-made Fenestrated Stent Graft to Preserve the Left Subclavian Artery
- Multislice CT Virtual Intravascular Endoscopy for Assessing Pulmonary Embolisms: a Pictorial Review
- Endovascular Treatment of Abdominal Aortic Aneurysm
- Fenestrated Stent Graft Repair of Abdominal Aortic Aneurysm: Hemodynamic Analysis of the Effect of Fenestrated Stents on the Renal Arteries