Korean J Radiol.
2010 Feb;11(1):95-106. 10.3348/kjr.2010.11.1.95.
Fenestrated Stent Graft Repair of Abdominal Aortic Aneurysm: Hemodynamic Analysis of the Effect of Fenestrated Stents on the Renal Arteries
- Affiliations
-
- 1Discipline of Medical Imaging, Department of Imaging and Applied Physics, Curtin University of Technology, Perth, Western Australia, Australia. z.sun@curtin.edu.au
- 2Department of Electronic Engineering, Faculty of Engineering, King Mongkut's Institute of Technology Ladkrabang, Bangkok, Thailand.
- KMID: 1787019
- DOI: http://doi.org/10.3348/kjr.2010.11.1.95
Abstract
OBJECTIVE
We wanted to investigate the hemodynamic effect of fenestrated stents on the renal arteries with using a fluid structure interaction method.
MATERIALS AND METHODS
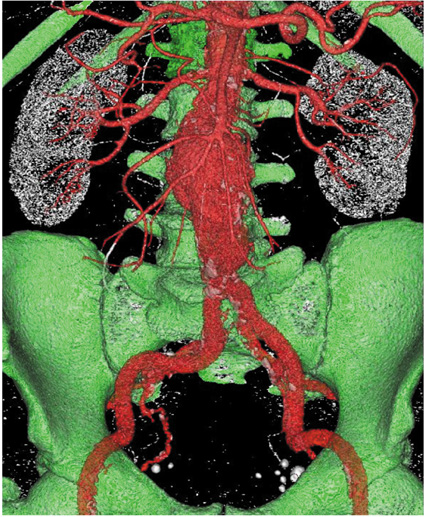
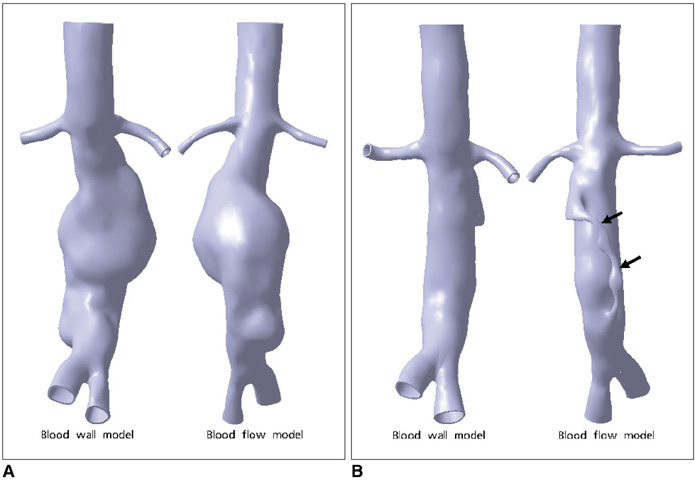
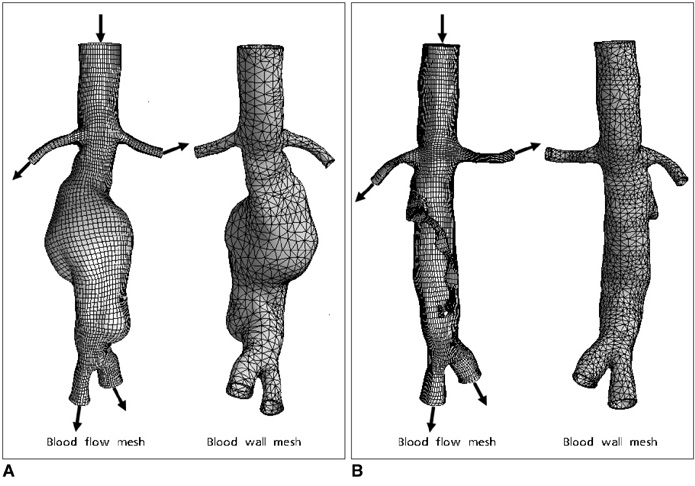
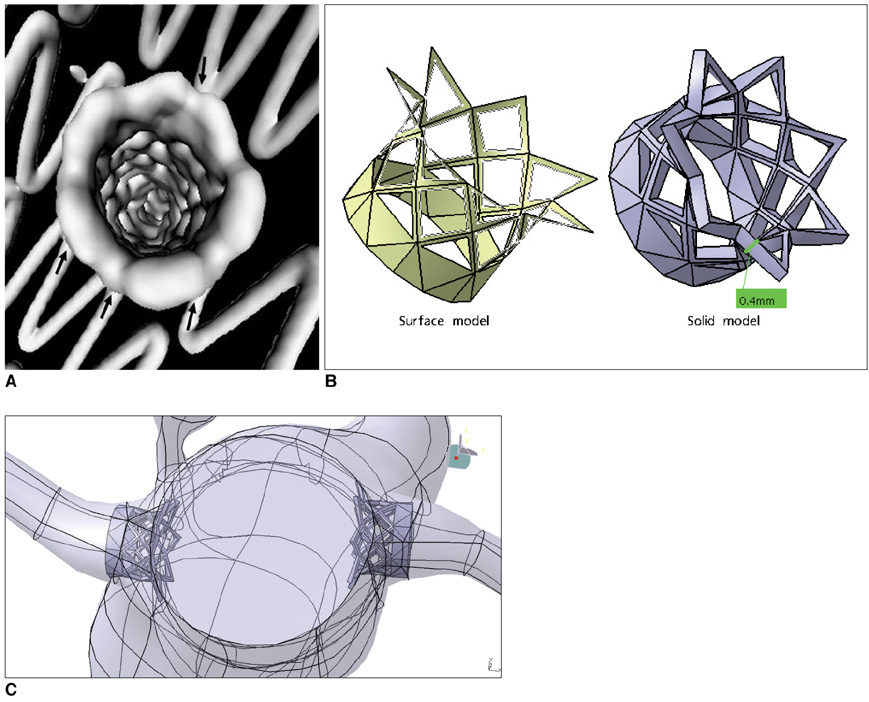
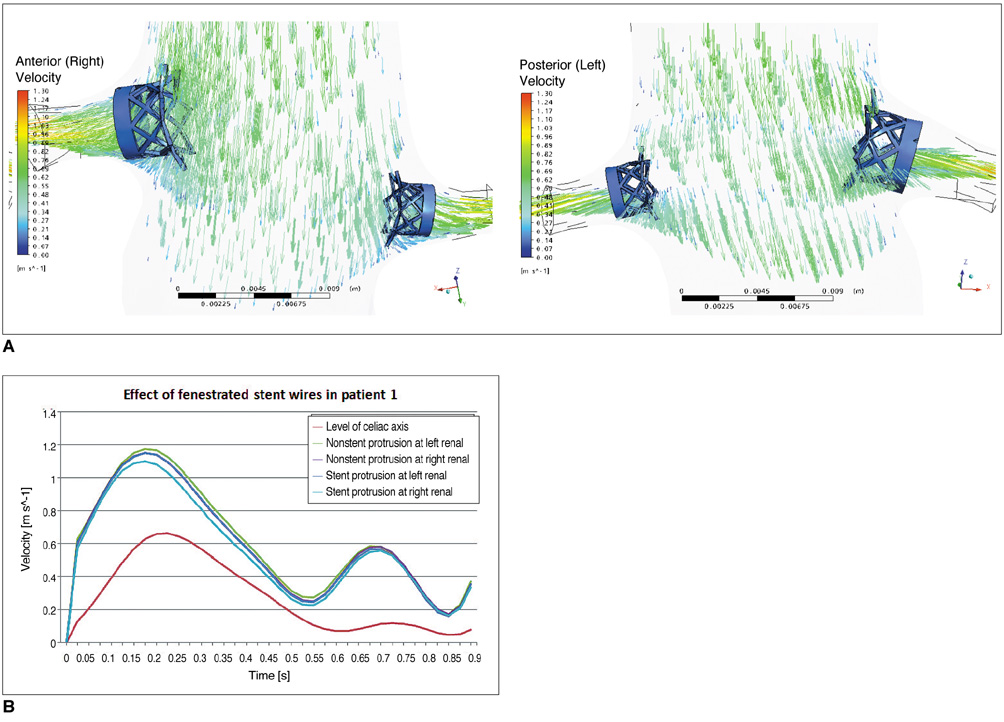
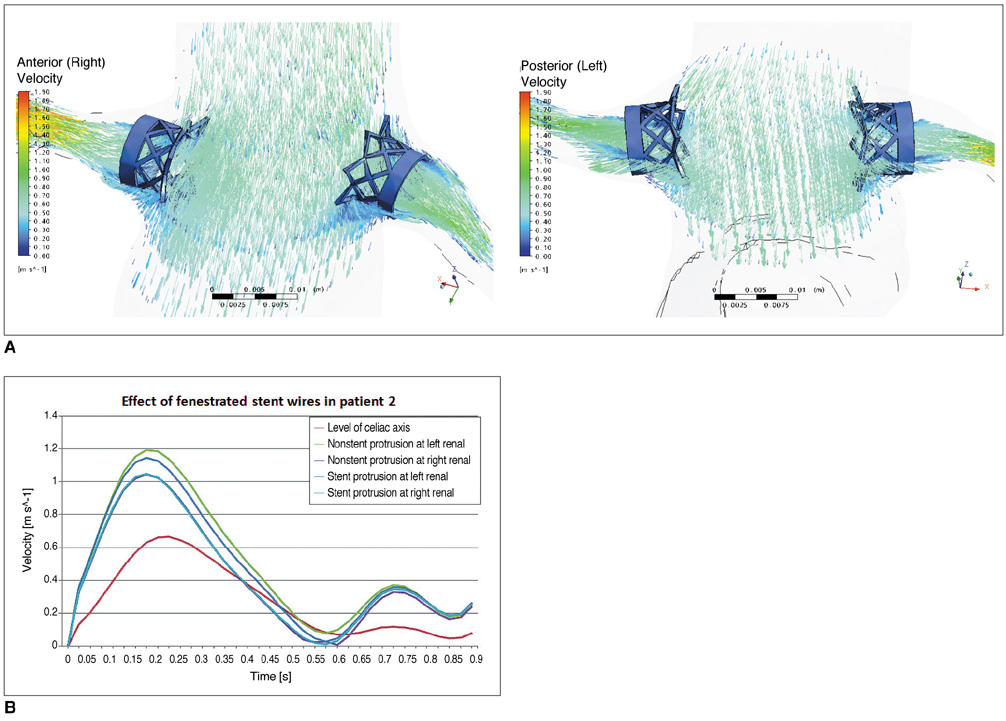
Two representative patients who each had abdominal aortic aneurysm that was treated with fenestrated stent grafts were selected for the study. 3D realistic aorta models for the main artery branches and aneurysm were generated based on the multislice CT scans from two patients with different aortic geometries. The simulated fenestrated stents were designed and modelled based on the 3D intraluminal appearance, and these were placed inside the renal artery with an intra-aortic protrusion of 5.0-7.0 mm to reflect the actual patients' treatment. The stent wire thickness was simulated with a diameter of 0.4 mm and hemodynamic analysis was performed at different cardiac cycles.
RESULTS
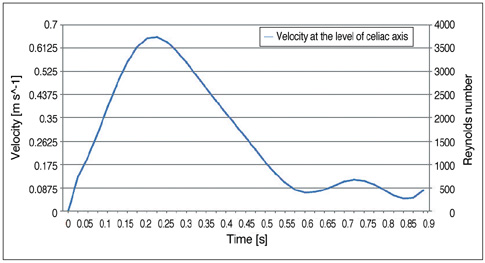
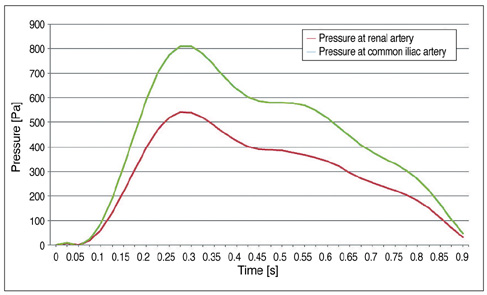
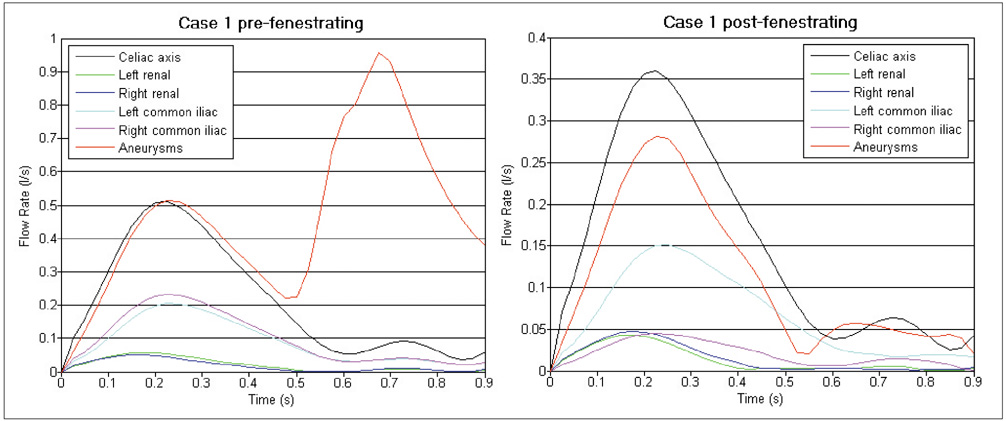
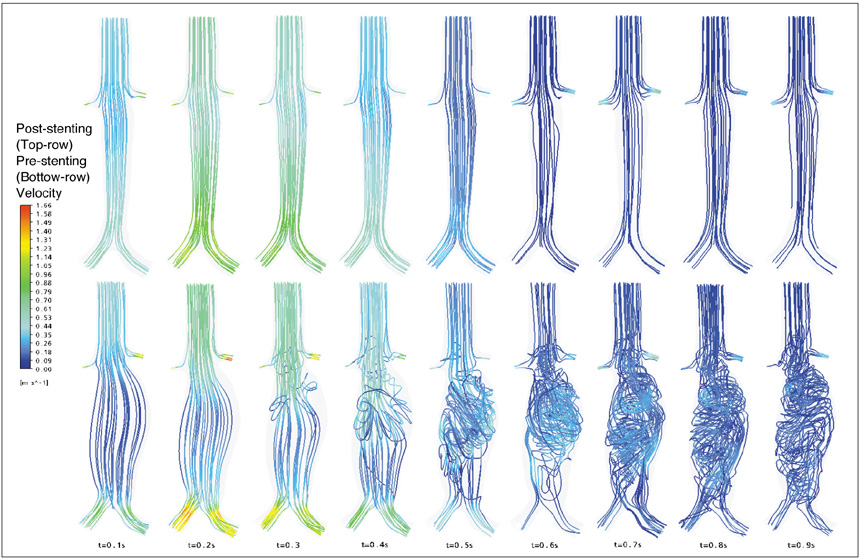
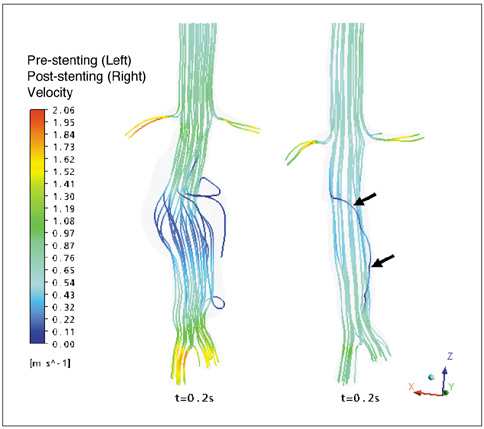
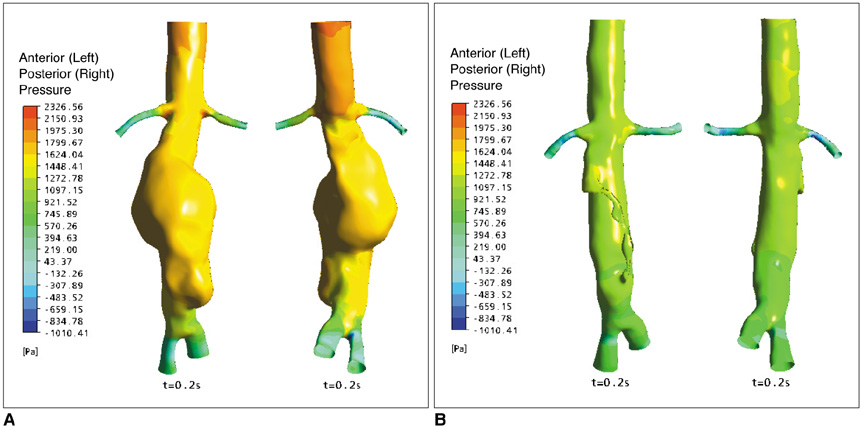
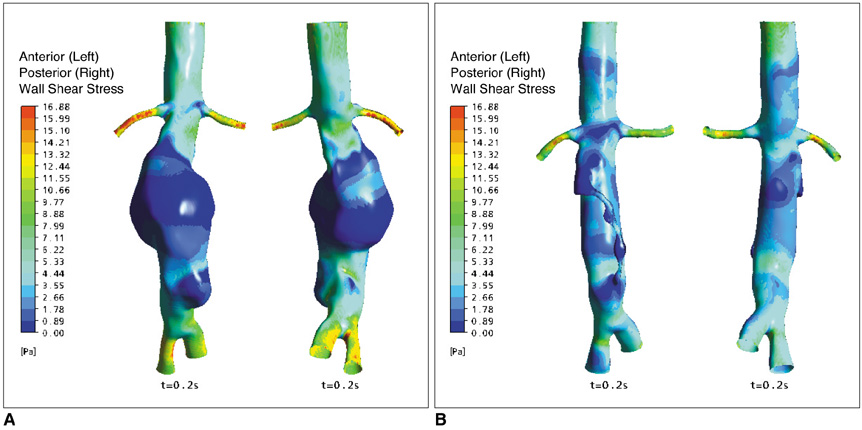
Our results showed that the effect of the fenestrated stent wires on the renal blood flow was minimal because the flow velocity was not significantly affected when compared to that calculated at pre-stent graft implantation, and this was despite the presence of recirculation patterns at the proximal part of the renal arteries. The wall pressure was found to be significantly decreased after fenestration, yet no significant change of the wall shear stress was noticed at post-fenestration, although the wall shear stress was shown to decrease slightly at the proximal aneurysm necks.
CONCLUSION
Our analysis demonstrates that the hemodynamic effect of fenestrated renal stents on the renal arteries is insignificant. Further studies are needed to investigate the effect of different lengths of stent protrusion with variable stent thicknesses on the renal blood flow, and this is valuable for understanding the long-term outcomes of fenestrated repair.
MeSH Terms
Figure
Reference
-
1. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004. 364:843–848.2. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004. 351:1607–1618.3. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991. 5:491–499.4. Buth J, van Marrewijk CJ, Harris PL, Hop WC, Riambau V, Laheij RJ. EUROSTSR Collaborators. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: a report on the EUROSTAR experience. J Vasc Surg. 2002. 35:211–221.5. Lobato AC, Quick RC, Vaughn PL, Rodriguez-Lopez J, Douglas M, Diethrich EB. Transrenal fixation of aortic endografts: intermediate follow-up of a single-center experience. J Endovasc Ther. 2000. 7:273–278.6. Bove PG, Long GW, Zelenock GB, Bendick PJ, Khoury MD, Burr MO, et al. Transrenal fixation of aortic stent-grafts for the treatment of infrarenal aortic aneurysmal disease. J Vasc Surg. 2000. 32:697–703.7. Green RM. Patient selection for endovascular abdominal aortic aneurysm repair. J Am Coll Surg. 2002. 194:S67–S73.8. Dillavou ED, Muluk SC, Rhee RY, Tzeng E, Woody JD, Gupta N, et al. Does hostile neck anatomy preclude successful endovascular aortic aneurysm repair? J Vasc Surg. 2003. 38:657–663.9. Browne TF, Hartley D, Purchas S, Rosenberg M, Van Schie G, Lawrence-Brown M. A fenestrated covered suprarenal aortic stent. Eur J Vasc Endovasc Surg. 1999. 18:445–449.10. Faruqi RM, Chuter TA, Reilly LM, Sawhney R, Wall S, Canto C, et al. Endovascular repair of abdominal aortic aneurysm using a pararenal fenestrated stent-graft. J Endovasc Surg. 1999. 6:354–358.11. Stanley BM, Semmens JB, Lawrence-Brown MM, Goodman MA, Hartley DE. Fenestration in endovascular grafts for aortic aneurysm repair: new horizons for preserving blood flow in branch vessels. J Endovasc Ther. 2001. 8:16–24.12. Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther. 2001. 8:3–15.13. Sun Z, Mwipatayi BP, Semmens JB, Lawrence-Brown MM. Short to midterm outcomes of fenestrated endovascular grafts in the treatment of abdominal aortic aneurysms: a systematic review. J Endovasc Ther. 2006. 13:747–753.14. Semmens JB, Lawrence-Brown MM, Hartley DE, Allen YB, Green R, Nadkarni S. Outcomes of fenestrated endografts in the treatment of abdominal aortic aneurysm in Western Australia (1997-2004). J Endovasc Ther. 2006. 13:320–329.15. Sun Z, Allen YB, Nadkarni S, Knight R, Hartley DE, Lawrence-Brown MM. CT virtual intravascular endoscopy in the visualization of fenestrated stent-grafts. J Endovasc Ther. 2008. 15:42–51.16. Sun Z, Allen YB, Mwipatayi BP, Hartley DE, Lawrence-Brown MM. Multislice CT angiography in the follow-up of fenestrated endovascular grafts: effect of slice thickness on 2D and 3D visualization of the fenestration stents. J Endovasc Ther. 2008. 15:417–426.17. Richter GM, Palmaz JC, Noeldge G, Tio F. Relationship between blood flow, thrombus, and neointima in stents. J Vasc Interv Radiol. 1999. 10:598–604.18. Beythien C, Gutensohn K, Bau J, Hamm CW, Kuhnl P, Meinertz T, et al. Influence of stent length and heparin coating on platelet activation: a flow cytometric analysis in a pulsed floating model. Thromb Res. 1999. 94:79–86.19. Peacock J, Hankins S, Jones T, Lutz R. Flow instabilities induced by coronary artery stents: assessment with an in vitro pulse duplicator. J Biomech. 1995. 28:17–26.20. Sun Z, Winder RJ, Kelly BE, Ellis PK, Hirst DG. CT virtual intravascular endoscopy of abdominal aortic aneurysms treated with suprarenal endovascular stent grafting. Abdom Imaging. 2003. 28:580–587.21. Sun Z. 3D multislice CT angiography in post-aortic stent grafting: a pictorial essay. Korean J Radiol. 2006. 7:205–211.22. Sun Z, Mwipatayi BP, Allen YB, Hartley DE, Lawrence-Brown MM. Multislice CT angiography of fenestrated endovascular stent grafting for treating abdominal aortic aneurysms: a pictorial review of the 2D/3D visualizations. Korean J Radiol. 2009. 10:285–293.23. Frauenfelder T, Lotfey M, Boehm T, Wildermuth S. Computational fluid dynamics: hemodynamic changes in abdominal aortic aneurysm after stent-graft implantation. Cardiovasc Intervent Radiol. 2006. 29:613–623.24. Borghi A, Wood NB, Mohiaddin RH, Xu XY. Fluid-solid interaction simulation of flow and stress pattern in thoracoabdominal aneurysms: a patient-specific study. J Fluids Struct. 2008. 24:270–280.25. Li Z, Kleinstreuer C. Analysis of biomechanical factors affecting stent-graft migration in an abdominal aortic aneurysm model. J Biomech. 2006. 39:2264–2273.26. Chong CK, How TV. Flow patterns in an endovascular stent-graft for abdominal aortic aneurysm repair. J Biomech. 2004. 37:89–97.27. Deplano V, Knapp Y, Bertrand E, Gaillard E. Flow behaviour in an asymmetric compliant experimental model for abdominal aortic aneurysm. J Biomech. 2007. 40:2406–2413.28. Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg. 2002. 36:589–597.29. Li Z, Kleinstreuer C. Fluid-structure interaction effects on sac-blood pressure and wall stress in a stented aneurysm. J Biomech Eng. 2005. 127:662–671.30. Liffman K, Lawrence-Brown MM, Semmens JB, Sutalo ID, Bui A, White F, et al. Suprarenal fixation: effect on blood flow of an endoluminal stent wire across an arterial orifice. J Endovasc Ther. 2003. 10:260–274.31. Carlier SG, van Damme LC, Blommerde CP, Wentzel JJ, van Langehove G, Verheye S, et al. Augmentation of wall shear stress inhibits neointimal hyperplasia after stent implantation: inhibition through reduction inflammation? Circulation. 2003. 107:2741–2746.32. Prati F, Di Mario C, Moussa I, Reimers B, Mallus MT, Parma A, et al. In-stent neointimal proliferation correlates with the amount of residual plaque burden outside the stent: an intravascular ultrasound study. Circulation. 1999. 99:1011–1014.33. Gaillard E, Bergeron P, Deplano V. Influence of wall compliance on hemodynamics in models of abdominal aortic aneurysm. J Endovasc Ther. 2007. 14:593–599.34. Li Z, Kleinstreuer C. Computational analysis of type II endoleaks in a stented abdominal aortic aneurysm model. J Biomech. 2006. 39:2573–2582.35. Lawrence-Brown MM, Sun Z, Semmens JB, Liffman K, Sutalo ID, Hartley DB. Type II endoleaks: when is intervention indicated and what is the index of suspicion for types I or III? J Endovasc Ther. 2009. 16:I106–I118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endovascular Repair of Thoracic Aortic Aneurysm Using a Custom-made Fenestrated Stent Graft to Preserve the Left Subclavian Artery
- Multislice CT Angiography of Fenestrated Endovascular Stent Grafting for Treating Abdominal Aortic Aneurysms: a Pictorial Review of the 2D/3D Visualizations
- Endovascular Treatment of Abdominal Aortic Aneurysm
- Anatomical feasibility of fenestrated stent graft to treat complex abdominal aortic aneurysms from a Korean single institute database
- Successful Customized Fenestrated Endovascular Aortic Aneurysm Repair in a Patient With Complex Abdominal Aortic Aneurysm