J Korean Med Sci.
2009 Aug;24(4):547-554. 10.3346/jkms.2009.24.4.547.
Comparison of Cytokine Expression in Mesenchymal Stem Cells from Human Placenta, Cord Blood, and Bone Marrow
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Korea University, Seoul, Korea. jh36640@hanmail.net
- 2Department of Obstetrics and Gynecology, School of Medicine, Pochon CHA University, Seoul, Korea.
- 3Women's Cancer Center Research Institute, School of Medicine, Korea University, Seoul, Korea.
- 4Research Center, RNL BIO CO., Ltd, Seoul, Korea.
- 5Department of Orthopedic Surgery, School of Medicine, Korea University, Seoul, Korea.
- KMID: 1779182
- DOI: http://doi.org/10.3346/jkms.2009.24.4.547
Abstract
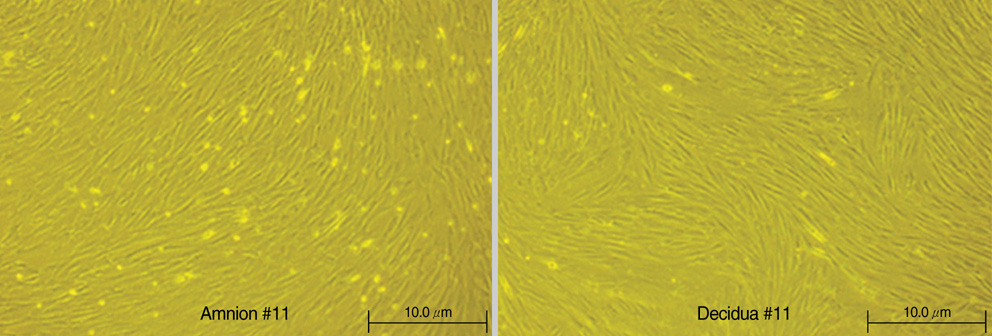
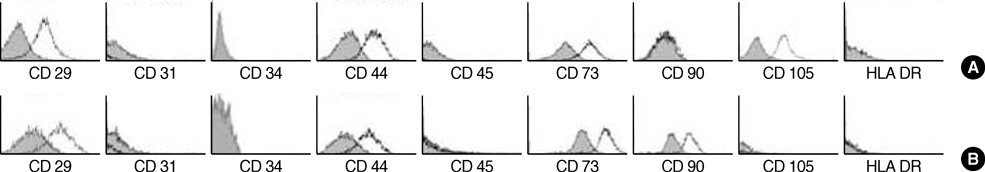
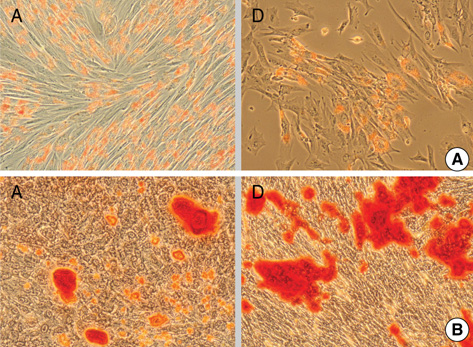
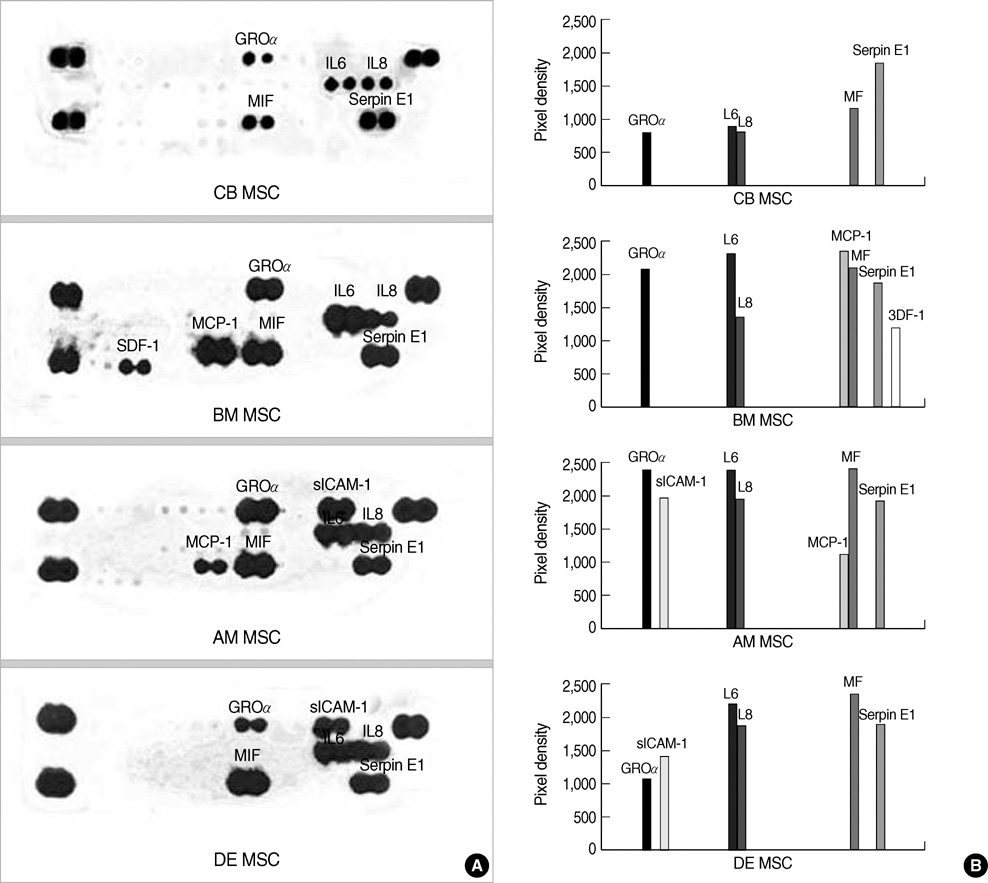
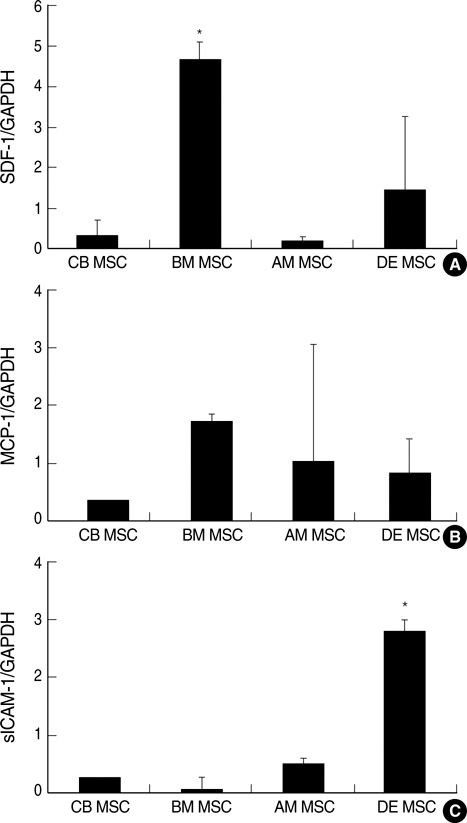
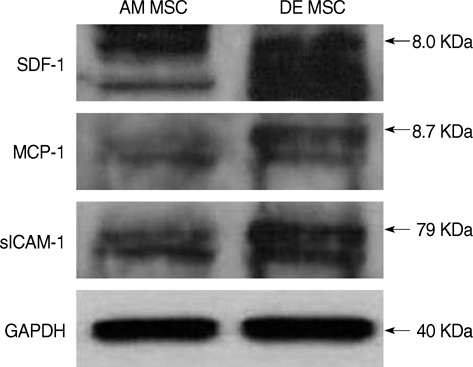
- Mesenchymal stem cells (MSCs) are capable of self-renewal and differentiation into lineages of mesenchymal tissues that are currently under investigation for a variety of therapeutic applications. The purpose of this study was to compare cytokine gene expression in MSCs from human placenta, cord blood (CB) and bone marrow (BM). The cytokine expression profiles of MSCs from BM, CB and placenta (amnion, decidua) were compared by proteome profiler array analysis. The cytokines that were expressed differently, in each type of MSC, were analyzed by real-time PCR. We evaluated 36 cytokines. Most types of MSCs had a common expression pattern including MIF (GIF, DER6), IL-8 (CXCL8), Serpin E1 (PAI-1), GROalpha(CXCL1), and IL-6. MCP-1, however, was expressed in both the MSCs from the BM and the amnion. sICAM-1 was expressed in both the amnion and decidua MSCs. SDF-1 was expressed only in the BM MSCs. Real-time PCR demonstrated the expression of the cytokines in each of the MSCs. The MSCs from bone marrow, placenta (amnion and decidua) and cord blood expressed the cytokines differently. These results suggest that cytokine induction and signal transduction are different in MSCs from different tissues.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Stemness Evaluation of Mesenchymal Stem Cells from Placentas According to Developmental Stage: Comparison to Those from Adult Bone Marrow
Hwa Jung Sung, Soon Cheol Hong, Ji Hyun Yoo, Jee Hyun Oh, Hye Jin Shin, In Young Choi, Ki Hoon Ahn, Sun Haeng Kim, Yong Park, Byung Soo Kim
J Korean Med Sci. 2010;25(10):1418-1426. doi: 10.3346/jkms.2010.25.10.1418.Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells
Jung Won Kang, Hye Cheong Koo, Sun Young Hwang, Sung Keun Kang, Jeong Chan Ra, Moon Han Lee, Yong Ho Park
J Vet Sci. 2012;13(1):23-31. doi: 10.4142/jvs.2012.13.1.23.
Reference
-
1. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article3. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004. 22:1338–1345.4. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article5. Gutierrez-Rodriguez M, Reyes-Maldonado E, Mayani H. Characterization of the adherent cells developed in Dexter-type long-term cultures from human umbilical cord blood. Stem Cells. 2000. 18:46–52.
Article6. Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006. 30:681–687.
Article7. Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006. 24:2202–2208.
Article8. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–848.
Article9. Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004. 22:415–427.
Article10. Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006. 24:23–33.
Article11. Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007. 25:1737–1745.
Article12. Gonzalez R, Maki CB, Pacchiarotti J, Csontos S, Pham JK, Slepko N, Patel A, Silva F. Pluripotent marker expression and differentiation of human second trimester mesenchymal stem cells. Biochem Biophys Res Commun. 2007. 362:491–497.
Article13. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.
Article14. Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, Krause DS. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003. 31:413–420.
Article15. Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Lowik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002. 30:870–878.
Article16. Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004. 363:1411–1412.
Article17. Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.
Article18. Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001. 122:713–734.
Article19. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004. 36:568–584.
Article20. in't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003. 88:845–852.21. Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003. 171:3426–3434.
Article22. Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996. 166:585–592.23. Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004. 4:360–370.
Article24. Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, Kishimoto T, Matsui Y, Nagasawa T. Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci USA. 2003. 100:5319–5323.
Article25. Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003. 362:697–703.
Article26. Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998. 95:9448–9453.
Article27. Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005. 280:35760–35766.
Article28. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006. 203:2201–2213.
Article29. Witkowska AM, Borawska MH. Soluble intracellular adhesion molecule-1 (sICAM-1) : an overview. Eur Cytokine Netw. 2004. 15:91–98.30. Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcelluar migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005. 106:584–592.31. Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005. 105:3793–3801.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cord Blood-Current Status and Perspective
- Mesenchymal Stem Cell Therapy in Pulmonary Disease
- Use of Cord Blood Stem Cells in Cell Therapy
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Neural Antigen Expressions in Cultured Human Umbilical Cord Blood Stem Cells in vitro