J Korean Med Sci.
2006 Jun;21(3):518-526. 10.3346/jkms.2006.21.3.518.
Corticotropin-releasing Factor (CRF) and Urocortin Promote the Survival of Cultured Cerebellar GABAergic Neurons Through the Type 1 CRF Receptor
- Affiliations
-
- 1Department of Anatomy, Center for Molecular Medicine, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Korea. khlee@med.skku.ac.kr
- 2School of Life Sciences and Biotechnology, Korea University, Seoul, Korea.
- 3Department of Anatomy, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 1778438
- DOI: http://doi.org/10.3346/jkms.2006.21.3.518
Abstract
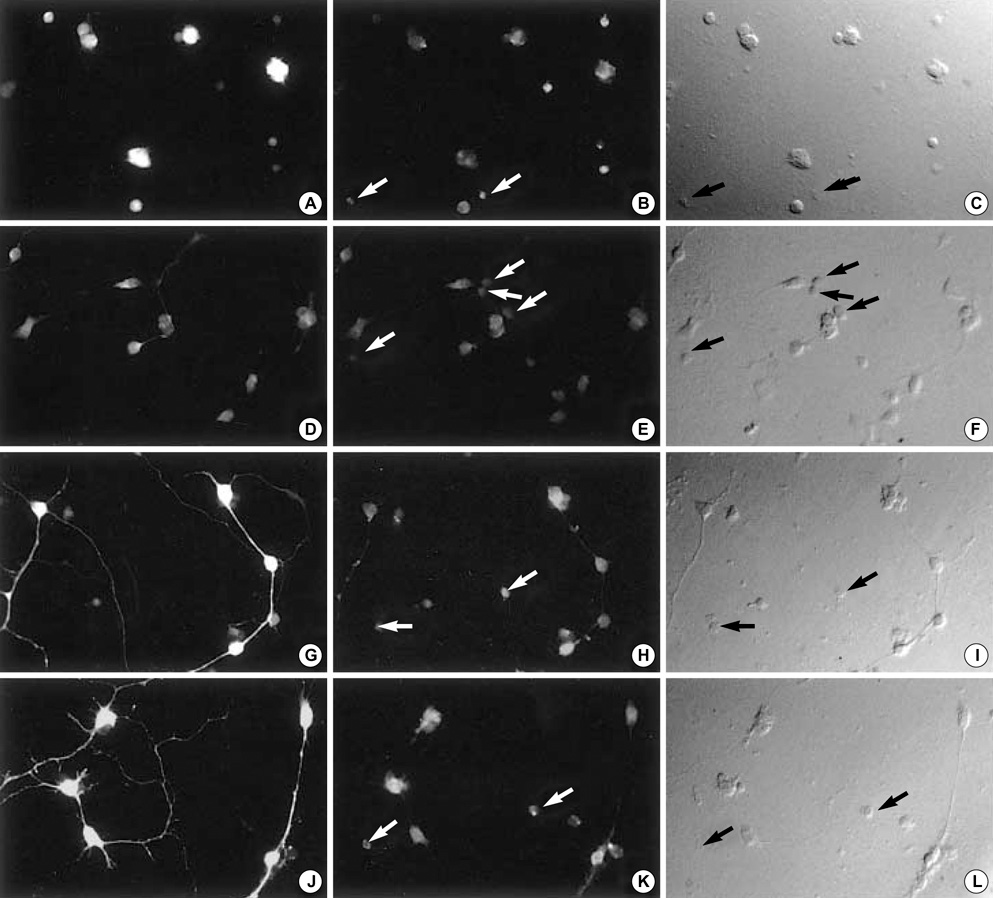
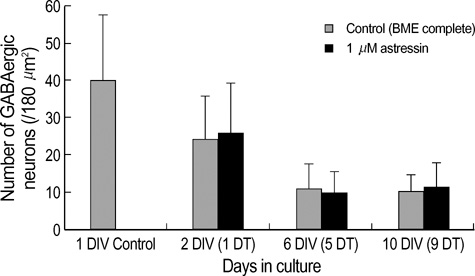
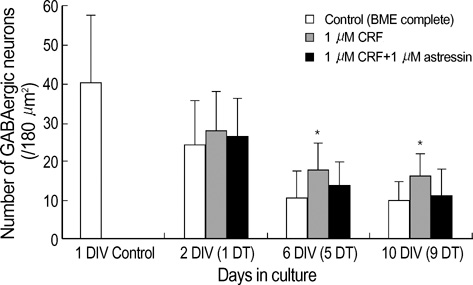
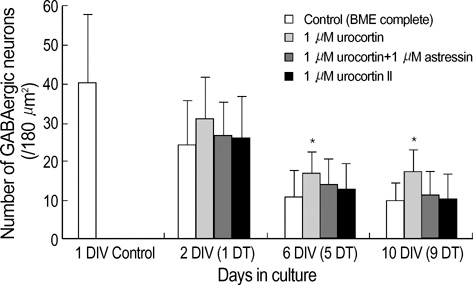
- Corticotropin releasing factor (CRF) is known to be involved in the stress response and in some degenerative brain disorders. In addition, CRF has a role as a neuromodulator in adult cerebellar circuits. Data from developmental studies suggest a putative role for CRF as a trophic factor during cerebellar development. In this study, we investigated the trophic role for CRF family of peptides by culturing cerebellar neurons in the presence of CRF, urocortin or urocortin II. Primary cell cultures of cerebella from embryonic day 18 mice were established, and cells were treated for either 1, 5 or 9 days with Basal Medium Eagles complete medium alone or complete medium with 1 micrometer CRF, urocortin, or urocortin II. The number of GABA-positive neurons in each treatment condition was counted at each culture age for monitoring the changes in neuronal survival. Treatment with 1 micrometer CRF or 1 micrometer urocortin increased the survival of GABAergic neurons at 6 days in vitro and 10 days in vitro, and this survival promoting effect was abolished by treatment with astressin in the presence of those peptides. Based on these data, we suggest that CRF or urocortin has a trophic role promoting the survival of cerebellar GABAergic neurons in cultures.
MeSH Terms
-
gamma-Aminobutyric Acid/*metabolism
Time Factors
Receptors, Corticotropin-Releasing Hormone/*metabolism
Peptides/chemistry
Neurons/*metabolism
Mice, Inbred C57BL
Mice
Immunohistochemistry
Image Processing, Computer-Assisted
Corticotropin-Releasing Hormone/biosynthesis/*physiology
Cerebellum/*embryology/*metabolism
Cells, Cultured
Cell Survival
Animals
Figure
Reference
-
1. Lezoualc'h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid beta precursor protein and with the suppression of nuclear factor-kappaB. Mol Endocrinol. 2000. 14:147–159.2. Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001. 22:733–741.
Article3. Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behaviora responses to stress. Peptides. 2001. 22:713–724.4. Bishop GA. Neuromodulatory effects of corticotropin releasing factor on cerebellar Purkinje cells: an in vivo study in the cat. Neuroscience. 1990. 39:251–257.
Article5. Fox EA, Gruol DL. Corticotropin-releasing factor suppresses the afterhyperpolarization in cerebellar Purkinje neurons. Neurosci Lett. 1993. 149:103–107.
Article6. King JS, Madtes P Jr, Bishop GA, Overbeck TL. The distribution of corticotropin-releasing factor (CRF), CRF binding sites and CRF1 receptor mRNA in the mouse cerebellum. Prog Brain Res. 1997. 114:55–66.7. Bishop GA, King JS. Corticotropin releasing factor in the embryonic mouse cerebellum. Exp Neurol. 1999. 160:489–499.
Article8. Overbeck TL, King JS. Developmental expression of corticotropin-releasing factor in the postnatal murine cerebellum. Brain Res Dev Brain Res. 1999. 115:145–159.
Article9. Madtes P Jr, King JS. The temporal and spatial development of CRF binding sites in the postnatal mouse cerebellum. Neurosci Res. 1999. 34:45–50.
Article10. Bishop GA. Development of a corticotropin-releasing factor-mediated effect on the firing rate of Purkinje cells in the postnatal mouse cerebellum. Exp Neurol. 2002. 178:165–174.
Article11. Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002. 23:71–77.
Article12. Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995. 136:4139–4142.
Article13. Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996. 17:166–172.
Article14. King JS, Bishop GA. The distribution and cellular localization of CRF-R1 in the vermis of the postnatal mouse cerebellum. Exp Neurol. 2002. 178:175–185.
Article15. King JS, Bishop GA. Localization of the type 1 corticotropin releasing factor receptor (CRF-R1) in the embryonic mouse cerebellum. J Neurocytol. 2003. 32:305–316.
Article16. Lee KH, Bishop GA, Tian JB, King JS. Evidence for an axonal localization of the type 2 corticotropin-releasing factor receptor during postnatal development of the mouse cerebellum. Exp Neurol. 2004. 187:11–22.
Article17. Fox MW, Anderson RE, Meyer FB. Neuroprotection by corticotropin releasing factor during hypoxia in rat brain. Stroke. 1993. 24:1072–1075.
Article18. Madtes P Jr, Lee KH, King JS, Burry RW. Corticotropin releasing factor enhances survival of cultured GABAergic cerebellar neurons after exposure to a neurotoxin. Brain Res Dev Brain Res. 2004. 151:119–128.
Article19. Ha BK, Bishop GA, King JS, Burry RW. Corticotropin releasing factor induces proliferation of cerebellar astrocytes. J Neurosci Res. 2000. 62:789–798.
Article20. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001. 98:2843–2848.
Article21. Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul Pept. 2000. 93:85–92.
Article22. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001. 98:7570–7575.
Article23. Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999. 415:285–312.
Article24. Fischer G. Cultivation of mouse cerebellar cells in serum free, hormonally defined media: survival of neurons. Neurosci Lett. 1982. 28:325–329.
Article25. Schilling K, Dickinson MH, Connor JA, Morgan JI. Electrical activity in cerebellar cultures determines Purkinje cell dendritic growth patterns. Neuron. 1991. 7:891–902.
Article26. Baader SL, Schilling K. Glutamate receptors mediate dynamic regulation of nitric oxide synthase expression in cerebellar granule cells. J Neurosci. 1996. 16:1440–1449.
Article27. Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999. 41:281–294.
Article28. De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995. 20:789–819.
Article29. Radulovic M, Hippel C, Spiess J. Corticotropin-releasing factor (CRF) rapidly suppresses apoptosis by acting upstream of the activation of caspases. J Neurochem. 2003. 84:1074–1085.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-facets of Corticotropin-releasing Factor in Modulating Inflammation and Angiogenesis
- The expression of corticotropin-releasing factor and its receptors in the spinal cord and dorsal root ganglion in a rat model of neuropathic pain
- Effects of Repeated Stress on Expression of Corticotropin Releasing Factor Type I and II Receptors
- Corticotropin-releasing Factor Changes the Phenotype and Function of Dendritic Cells in Mouse Mesenteric Lymph Nodes
- Efficacy of Maternal Serum Corticotropin Releasing Factor Levels in Diagnosis of Pregnancies Complicated by Preterm Labor