J Korean Med Sci.
2007 Jun;22(3):502-507. 10.3346/jkms.2007.22.3.502.
The Efficacy of an Acrylic Intraocular Lens Surface Modified with Polyethylene Glycol in Posterior Capsular Opacification
- Affiliations
-
- 1Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea. wrwee@snu.ac.kr
- 2Department of Ophthalmology, College of Medicine, Chung-Ang University, Seoul, Korea.
- 3Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1778361
- DOI: http://doi.org/10.3346/jkms.2007.22.3.502
Abstract
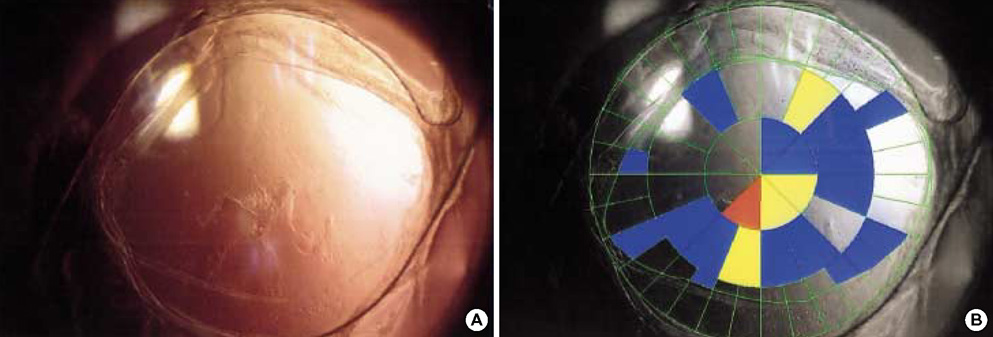
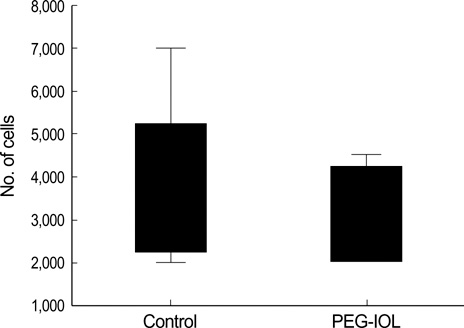
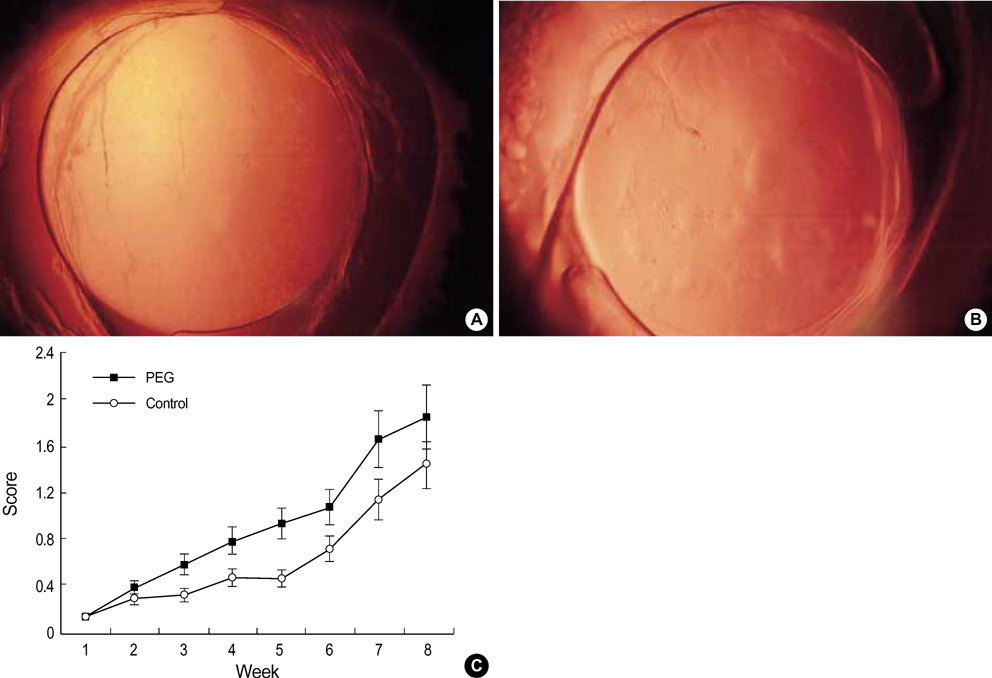
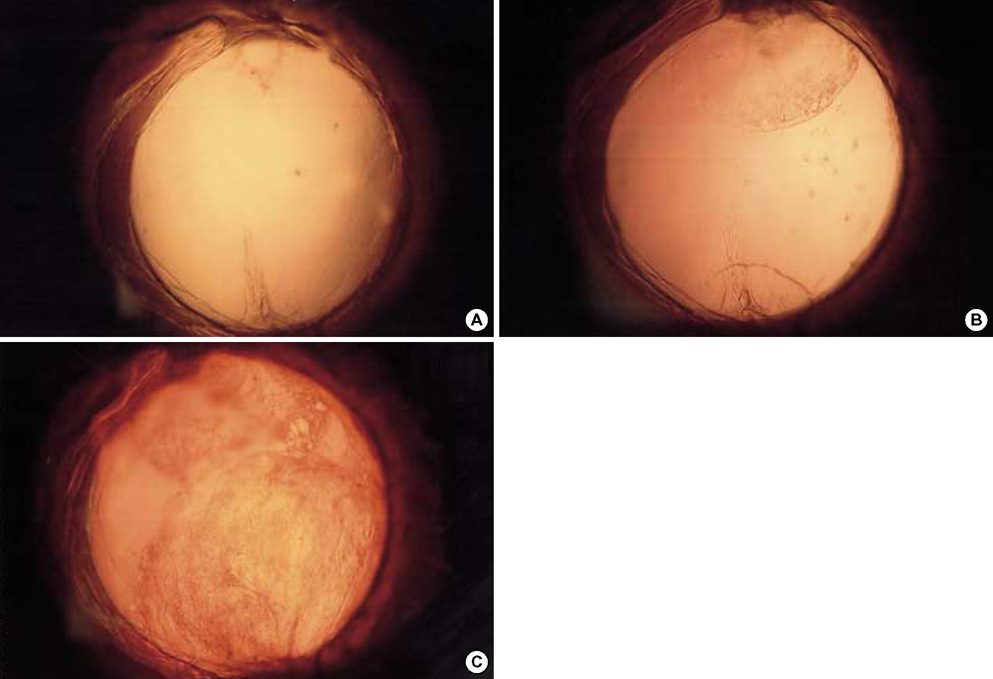
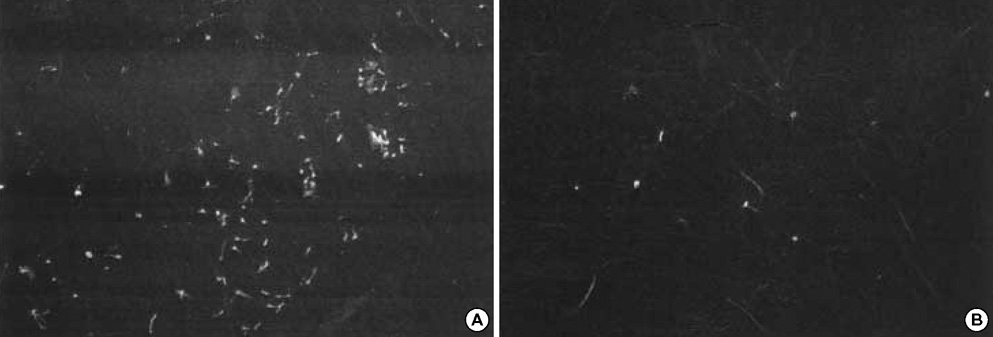
- To investigate if the surface modification of intraocular lens (IOL) is efficient in the prevention of posterior capsular opacification (PCO), the acrylic surface of intraocular lens (Acrysof(R)) was polymerized with polyethylene glycol (PEG-IOL). The human lens epithelial cells (1x10(4) cells/mL) were inoculated on PEG grafted or unmodified acrylic lenses for the control. The adherent cells on each IOL surface were trypsinized and counted. The every PEG-IOL was implanted in 20 New Zealand rabbits after removal of crystalline lens. The formations of PCO were checked serially through retroilluminated digital photography, and the severity scores were calculated using POCOman(R). The cell adherence patterns on each IOL were examined by scanning electron microscopy. As a result, the mean number of adherent cells of PEG-IOL (3.2+/-1.1x10(3)) tended to be smaller than that of the acrylic controls (3.6+/-1.9x10(3)) without a statistical significance (p=0.73). However, the mean severity of PCO formation in PEG-IOL was significantly lower than that in the control during the third to sixth weeks after surgery. Scanning electron microscopy revealed that the more patch-like cells were found firmly attached to the IOL surface in control than in the PEG-IOL. Conclusively, PEG polymerization to the acrylic IOL would possibly lessen the formation of PCO after cataract removal.
Keyword
MeSH Terms
Figure
Reference
-
1. Peng Q, Apple DJ, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, Schoderbek R, Guindi A. Surgical prevention of posterior capsule opacification. Part 2: enhancement of cortical cleanup by focusing on hydrodissection. J Cataract Refract Surg. 2000. 26:188–197.2. Linnola RJ, Werner L, Pandey SK, Escobar-Gomez M, Znoiko SL, Apple DJ. Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. Part 2: explanted intraocular lenses. J Cataract Refract Surg. 2000. 26:1807–1818.3. Johnston RL, Spalton DJ, Hussain A, Marshall J. In vitro protein adsorption to 2 intraocular lens materials. J Cataract Refract Surg. 1999. 25:1109–1115.
Article4. Nishi O, Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg. 1999. 25:521–526.
Article5. Nishi O, Nishi K, Menapace R. Capsule-bending ring for the prevention of capsular opacification: a preliminary report. Ophthalmic Surg Lasers. 1998. 29:749–753.
Article6. Nishi O, Nishi K, Sakanishi K. Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthalmic Surg Lasers. 1998. 29:587–594.
Article7. Tanaka T, Yamakawa N, Mizusawa T, Usui M. Interaction between inflammatory cells and heparin-surface-modified intraocular lens. J Cataract Refract Surg. 2000. 26:1409–1412.
Article8. Trocme SD, Li H. Heparin-Surface-Modified Lens Study Group. Effect of heparin-surface-modified intraocular lenses on postoperative inflammation after phacoemulsification: a randomized trial in a United States patient population. Ophthalmology. 2000. 107:1031–1037.9. Yuan Z, Sun H, Yuan J. A 1-year study on carbon, titanium surface-modified intraocular lens in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2004. 242:1008–1013.
Article10. Lundvall A, Zetterstrom C, Lundgren B, Kugelberg U. Effect of 3-piece AcrySof and downsized heparin-surface-modified poly (methyl-methacrylate) intraocular lenses in infant rabbit eyes. J Cataract Refract Surg. 2003. 29:159–163.11. Gatinel D, Lebrun T, Le Toumelin P, Chaine G. Aqueous flare induced by heparin-surface-modified poly(methyl methacrylate) and acrylic lenses implanted through the same-size incision in patients with diabetes. J Cataract Refract Surg. 2001. 27:855–860.
Article12. Kim MK, Park IS, Park HD, Wee WR, Lee JH, Park KD, Kim SH, Kim YH. Effect of poly (ethylene glycol) graft polymerization of poly (methyl methacrylate) on cell adhesion. In vitro and in vivo study. J Cataract Refract Surg. 2001. 27:766–774.13. Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trial. Am J Ophthalmol. 1999. 128:271–279.
Article14. Fernandez V, Fragoso MA, Billotte C, Lamar P, Orozco MA, Dubovy S, Willcox M, Parel JM. Efficacy of various drugs in the prevention of posterior capsule opacification: experimental study of rabbit eyes. J Cataract Refract Surg. 2004. 30:2598–2605.
Article15. Smith SR, Daynes T, Hinckley M, Wallin TR, Olson RJ. The effect of lens edge design versus anterior capsule overlap on posterior capsule opacification. Am J Ophthalmol. 2004. 138:521–526.
Article16. Nishi O, Nishi K, Osakabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg. 2004. 30:2170–2176.17. Desai NP, Hubbell JA. Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J Biomed Mater Res. 1991. 25:829–843.
Article18. Lee JH, Kopecek J, Andrade JD. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J Biomed Mater Res. 1989. 23:351–368.
Article19. Han DK, Park KD, Ryu GH, Kim UY, Min BG, Kim YH. Plasma protein adsorption to sulfonated poly (ethylene oxide)-grafted polyurethane surface. J Biomed Mater Res. 1996. 30:23–30.20. Kim CK, Lee JA, Tchah HW. Adhesion and morphologic change of lens epithelial cell according to materials of intraocular lens. J Korean Ophthalmol Soc. 2003. 44:445–453.21. Lane SS, Burgi P, Milios GS, Orchowski MW, Vaughan M, Schwarte E. Comparison of the biomechanical behavior of foldable intraocular lenses. J Cataract Refract Surg. 2004. 30:2397–2402.
Article22. Kim TW, Kwon JW, Lee JH. Long-term follow-up of the insertion of capsular tension ring in cataract surgery. J Korean Ophthalmol Soc. 2005. 46:1624–1629.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Polyethylene Glycol Polymerization onto a Foldable Intraocular Lens in Pathogenesis of Posterior Capsular Opacity
- Posterior Capsule Opacification and Intraocular Lens Design with In-the-bag fixated Posterior Chamber Lens
- Clinical Results of Centerflex(R) Intraocular Ocular Lenses and Acrysof(R) Intraocular Ocular Lenses in Bilateral Cataracts
- Late Opacification of a Hydrophilic Acrylic Monofocal Intraocular Lens with Hydrophobic Surface after Vitrectomy
- Acrysof(R) and Polymethylmethacrylate Intraocular Lens Implantation in Diabetic Patients