J Korean Med Sci.
2011 Jan;26(1):33-41. 10.3346/jkms.2011.26.1.33.
Treatment Outcome and Mortality among Patients with Multidrug-resistant Tuberculosis in Tuberculosis Hospitals of the Public Sector
- Affiliations
-
- 1Clinical Research Center, National Masan Tuberculosis Hospital, Masan, Korea. sooli10@hanmail.net
- 2Department of Thoracic Medicine, Seobuk Hospital, Seoul, Korea.
- 3Department of Thoracic Surgery, National Mokpo Tuberculosis Hospital, Mokpo, Korea.
- 4Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 5Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1777973
- DOI: http://doi.org/10.3346/jkms.2011.26.1.33
Abstract
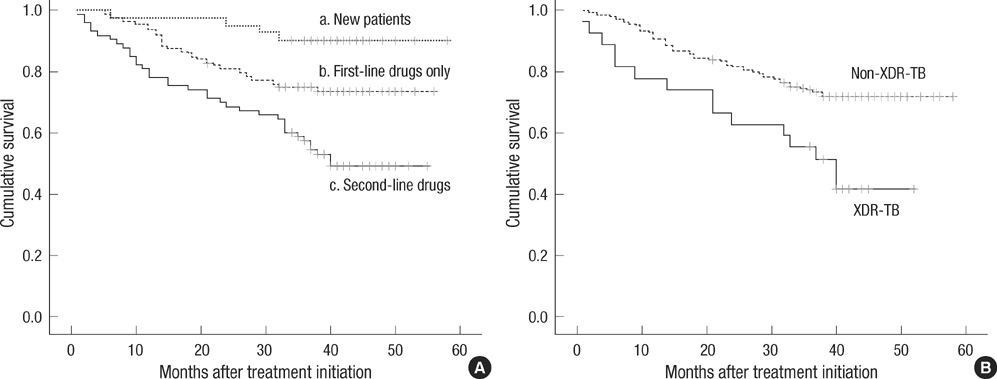
- This study was conducted to evaluate treatment outcome, mortality, and predictors of both in patients with multidrug-resistant tuberculosis (MDR-TB) at 3 TB referral hospitals in the public sector of Korea. We included MDR-TB patients treated at 3 TB referral hospitals in 2004 and reviewed retrospectively their medical records and mortality data. Of 202 MDR-TB patients, 75 (37.1%) had treatment success and 127 (62.9%) poor outcomes. Default rate was high (37.1%, 75/202), comprising 59.1% of poor outcomes. Male sex (adjusted odds ratio [aOR], 2.91; 95% confidence interval [CI], 1.13-7.49), positive smear at treatment initiation (aOR, 5.50; 95% CI, 1.22-24.90), and extensively drug-resistant TB (aOR, 10.72; 95% CI, 1.23-93.64) were independent predictors of poor outcome. The all-cause mortality rate was 31.2% (63/202) during the 3-4 yr after treatment initiation. In conclusion, the treatment outcomes of patients with MDR-TB at the 3 TB hospitals are poor, which may reflect the current status of MDR-TB in the public sector of Korea. A more comprehensive program against MDR-TB needs to be integrated into the National Tuberculosis Program of Korea.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Antitubercular Agents/*therapeutic use
Demography
Drug Resistance, Multiple, Bacterial
Drug Therapy, Combination
Female
Hospitals, Chronic Disease
Humans
Male
Middle Aged
Odds Ratio
Predictive Value of Tests
Retrospective Studies
Sex Factors
Treatment Outcome
Tuberculosis, Multidrug-Resistant/drug therapy/*mortality
Figure
Cited by 1 articles
-
Rapid Diagnosis of Tuberculosis and Multidrug Resistance Using a MGIT 960 System
Won-Jung Koh, Yousang Ko, Chang-Ki Kim, Kyung Sun Park, Nam Yong Lee
Ann Lab Med. 2012;32(4):264-269. doi: 10.3343/alm.2012.32.4.264.
Reference
-
1. Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR Jr. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993. 328:527–532.2. Kang YA, Choi YJ, Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Cost of treatment for multidrug-resistant tuberculosis in South Korea. Respirology. 2006. 11:793–798.3. World Health Organization. Publication No. WHO/HTM/TB/2006.361. Guidelines for the programmatic management of drug-resistant tuberculosis. 2006. Geneva, Switzerland: World Health Organization.4. Korean Centers for Disease Control and Prevention. 2008 Annual Report on the Notified Tuberculosis Patients in Korea. 2009. Seoul: Korean Centers for Disease Control and Prevention.5. Seung KJ, Bai GH, Kim SJ, Lew WJ, Park SK, Kim JY. The treatment of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2003. 7:912–919.6. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, Kim EK, Lee KM, Lee SS, Park JS, Koh WJ, Lee CH, Kim JY, Shim TS. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008. 178:1075–1082.7. Centers for Disease Control and Prevention. Revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep. 2006. 55:1176.8. National Tuberculosis Association. Diagnostic standards and classification of tuberculosis. 1961. New York: The Association.9. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998. 2:27–36.10. Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Lee NY, Park YK, Bai GH, Koh WJ. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007. 22:677–681.11. Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009. 24:592–595.12. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, Lee JK, Kim SJ. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–576.13. Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006. 55:301–305.14. Kim HJ, Hong YP, Kim SJ, Lew WJ, Lee EG. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis. 2001. 5:1129–1136.15. Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004. 8:361–368.16. Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007. 45:1290–1295.17. Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, Choi YS, Kim K, Kim J, Shim YM, Koh WJ. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008. 47:496–502.18. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009. 9:153–161.19. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009. 4:e6914.20. Chengsorn N, Bloss E, Anekvorapong R, Anuwatnonthakate A, Wattanaamornkiat W, Komsakorn S, Moolphate S, Limsomboon P, Kaewsaard S, Nateniyom S, Kanphukiew A, Varma JK. Tuberculosis services and treatment outcomes in private and public health care facilities in Thailand, 2004-2006. Int J Tuberc Lung Dis. 2009. 13:888–894.21. Chan ED, Laurel V, Strand MJ, Chan JF, Huynh ML, Goble M, Iseman MD. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004. 169:1103–1109.22. Jassal M, Bishai WR. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009. 9:19–30.23. Raviglione MC, Smith IM. XDR tuberculosis-implications for global public health. N Engl J Med. 2007. 356:656–659.24. Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, Fitzmaurice GM, Alcantara Viru FA, Appleton SC, Bayona JN, Bonilla CA, Chalco K, Choi S, Franke MF, Fraser HS, Guerra D, Hurtado RM, Jazayeri D, Joseph K, Llaro K, Mestanza L, Mukherjee JS, Muñoz M, Palacios E, Sanchez E, Sloutsky A, Becerra MC. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008. 359:563–574.25. Bonilla CA, Crossa A, Jave HO, Mitnick CD, Jamanca RB, Herrera C, Asencios L, Mendoza A, Bayona J, Zignol M, Jaramillo E. Management of extensively drug-resistant tuberculosis in Peru: cure is possible. PLoS ONE. 2008. 3:e2957.26. Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Atwood S, Mukherjee JS, Rich ML, Furin JJ, Nardell EA, Kim JY, Shin SS. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008. 372:1403–1409.27. Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006. 6:710–725.28. World Health Organization. Publication No. WHO/HTM/TB/2009.426. Global tuberculosis control: a short update to the 2009 report. 2009. Geneva, Switzerland: World Health Organization.29. Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Lomakina OB, Kourbatova EV. Social support and incentives programme for patients with tuberculosis: experience from the Russian Federation. Int J Tuberc Lung Dis. 2007. 11:1210–1215.30. Choi BY. Perspectives of policies on HIV/AIDS and tuberculosis control in Korea. Korean J Epidemiol. 2006. 28:75–84.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The survey for clinical course of intractable pulmonary tuberculosis
- Drug Resistance Patterns of Multidrug- and Extensively Drug-Resistant Tuberculosis in Korea: Amplification of Resistance to Oral Second-line Drugs
- Multidrug-resistant Tuberculosis Spondylitis: A Case Report
- WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update: Applicability in South Korea
- Issues Related to the Updated 2014 Korean Guidelines for Tuberculosis