J Korean Med Sci.
2011 Aug;26(8):1007-1013. 10.3346/jkms.2011.26.8.1007.
Association between Genetic Polymorphisms of CYP2D6 and Outcomes in Breast Cancer Patients with Tamoxifen Treatment
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. bwpark@yuhs.ac
- 2Department of Pharmacology and Pharmacogenomics Research Center, Inje University College of Medicine, Busan, Korea.
- 3Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1777838
- DOI: http://doi.org/10.3346/jkms.2011.26.8.1007
Abstract
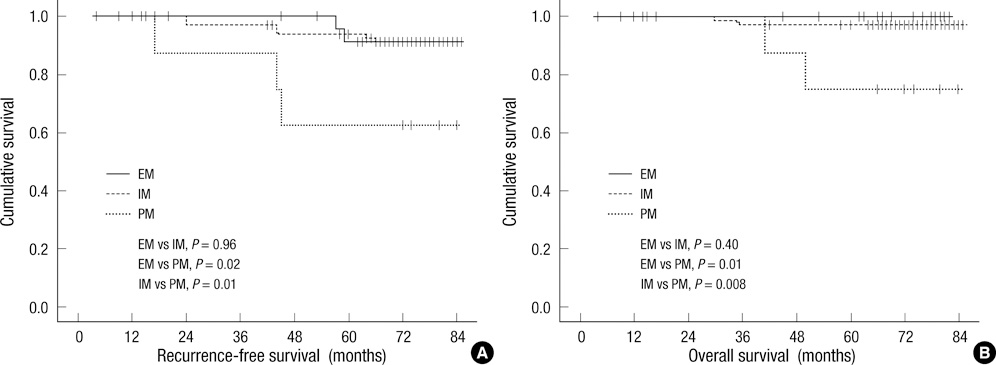
- The aim of the study was to evaluate the association between genetic polymorphisms of CYP2D6 and outcomes in breast cancer patients with tamoxifen treatment. We evaluated the CYP2D6 genetic polymorphisms in 766 breast cancer patients. Among them, 110 patients whose samples were prospectively collected before surgery and treated with tamoxifen were included to evaluate the association between CYP2D6 and outcomes. The genotypes of CYP2D6 were categorized as extensive metabolizer (EM), intermediate metabolizer (IM), and poor metabolizer (PM) according to the activity score. The clinicopathologic features of 110 patients were not significantly different among the three groups except for the T-stage and nodal status. The high T-stage and axillary metastasis were more frequent in the PM group. While recurrence-free and overall survival in the PM group was poorer than the other groups, there was no significant difference between the EM and the IM group. The difference between the PM and the other groups on univariate analysis disappeared on multivariate analysis. These conflicting results suggest that the clinical value of CYP2D6 polymorphisms is still unclear and more large-sized and comprehensively designed trials are necessary.
Keyword
MeSH Terms
Figure
Reference
-
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005. 365:1687–1717.2. Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004. 310:1062–1075.3. Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, Skaar TC. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006. 318:503–512.4. Borgna JL, Rochefort H. Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem. 1981. 256:859–868.5. Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004. 85:151–159.6. Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005. 55:471–478.7. Lim HS, Lee HJ, Lee KS, Lee ES, Jang IJ, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007. 25:3837–3845.8. Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008. 83:234–242.9. Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005. 23:9312–9318.10. Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM, Simon W, Eichelbaum M, Brauch H. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007. 25:5187–5193.11. Lee SJ, Lee SS, Jung HJ, Kim HS, Park SJ, Yeo CW, Shin JG. Discovery of novel functional variants and extensive evaluation of CYP2D6 genetic polymorphisms in Koreans. Drug Metab Dispos. 2009. 37:1464–1470.12. Li H, Feng L, Xu Y, Yao L, Ouyang T, Li J, Wang T, Fan Z, Lin B, Xie Y. The association of CYP2D6 *10 polymorphism with breast cancer risk and clinico-pathologic characteristics in Chinese women. Acta Oncol. 2006. 45:597–601.13. Xu Y, Sun Y, Yao L, Shi L, Wu Y, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, He L, Li P, Xie Y. Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol. 2008. 19:1423–1429.14. Okishiro M, Taguchi T, Kim SJ, Shimazu K, Tamaki Y, Noguchi S. Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer. 2009. 115:952–961.15. Gaedigk A, Gotschall RR, Forbes NS, Simon SD, Kearns GL, Leeder JS. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics. 1999. 9:669–682.16. Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, James LP, Wilson JT, Kearns GL, Leeder JS. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007. 81:510–516.17. Henry NL, Rae JM, Li L, Azzouz F, Skaar TC, Desta Z, Sikora MJ, Philips S, Nguyen AT, Storniolo AM, Hayes DF, Flockhart DA, Stearns V. Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009. 117:571–575.18. Ramón y Cajal T, Altés A, Paré L, del Rio E, Alonso C, Barnadas A, Baiget M. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat. 2010. 119:33–38.19. Laforest L, Wikman H, Benhamou S, Saarikoski ST, Bouchardy C, Hirvonen A, Dayer P, Husgafvel-Pursiainen K. CYP2D6 gene polymorphism in caucasian smokers: lung cancer susceptibility and phenotype-genotype relationships. Eur J Cancer. 2000. 36:1825–1832.20. Wolf CR, Smith CA, Bishop T, Forman D, Gough AC, Spurr NK. CYP2D6 genotyping and the association with lung cancer susceptibility. Pharmacogenetics. 1994. 4:104–106.21. Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997. 60:284–295.22. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002. 3:229–243.23. Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M, Tanigawara Y, Flockhart DA, Desta Z, Skaar TC, Aki F, Hirata K, Takatsuka Y, Okazaki M, Ohsumi S, Yamakawa T, Sasa M, Nakamura Y, Zembutsu H. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010. 28:1287–1293.24. Wegman P, Elingarami S, Carstensen J, Stål O, Nordenskjöld B, Wingren S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007. 9:R7.25. Park IH, Ro J, Park S, Lim HS, Lee KS, Kang HS, Jung SY, Lee S. Lack of any association between functionally significant CYP2D6 polymorphisms and clinical outcomes in early breast cancer patients receiving adjuvant tamoxifen treatment. Breast Cancer Res Treat. 2011. Doi: 10.1007/s10549-011-1425-2.26. Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, Salter J, Pineda S, Cuzick J, Dowsett M. Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endpoints in the ATAC Trial. Program and abstracts of the 33rd Annual San Antonio Breast Cancer Symposium. Cancer Res. 2010. 70:S1-7.27. Leyland-Jones B, Regan MM, Bouzyk M, Kammler R, Tang W, Pagani O, Malbach R, Dell'Orto P, Thürlimann B, Price KN, Viale G. Outcome according to CYP2D6 Genotype among postmenopausal women with endocrine-responsive early invasive breast cancer randomized in the BIG 1-98 Trial. Program and abstracts of the 33rd Annual San Antonio Breast Cancer Symposium. Cancer Res. 2010. 70:S1-8.28. Seruga B, Amir E. Cytochrome P450 2D6 and outcomes of adjuvant tamoxifen therapy: results of a meta-analysis. Breast Cancer Res Treat. 2010. 122:609–617.29. Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, Eddy SM, Dostalik J, Mount J, Azuma J, Fujio Y, Jang IJ, Shin SG, Bleavins MR, Williams JA, Paulauskis JD, Wilner KD. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther. 2008. 84:347–361.30. Dezentjé VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP, Casparie MK, Egberts AC, Nortier JW, Guchelaar HJ. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010. 28:2423–2429.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Antidepressants in Patients with Breast Cancer Taking Tamoxifen
- Toremifene, an Alternative Adjuvant Endocrine Therapy, Is Better Than Tamoxifen in Breast Cancer Patients with CYP2D6*10 Mutant Genotypes
- Adjuvant Hormonal Therapy: Current Standard and Practical Issues
- Tamoxifen Resistance and Crosstalk of Signal Transduction in Breast Cancer
- No Association of CYP2D6*4 and CYP2D6*10 Polymorphisms with Tardive Dyskinesia in Korean Schizophrenics