J Korean Med Sci.
2011 Aug;26(8):1001-1006. 10.3346/jkms.2011.26.8.1001.
Evaluation of Intratumoral HER-2 Heterogeneity by Fluorescence In Situ Hybridization in Invasive Breast Cancer: A Single Institution Study
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. kjs1976@yuhs.ac
- KMID: 1777837
- DOI: http://doi.org/10.3346/jkms.2011.26.8.1001
Abstract
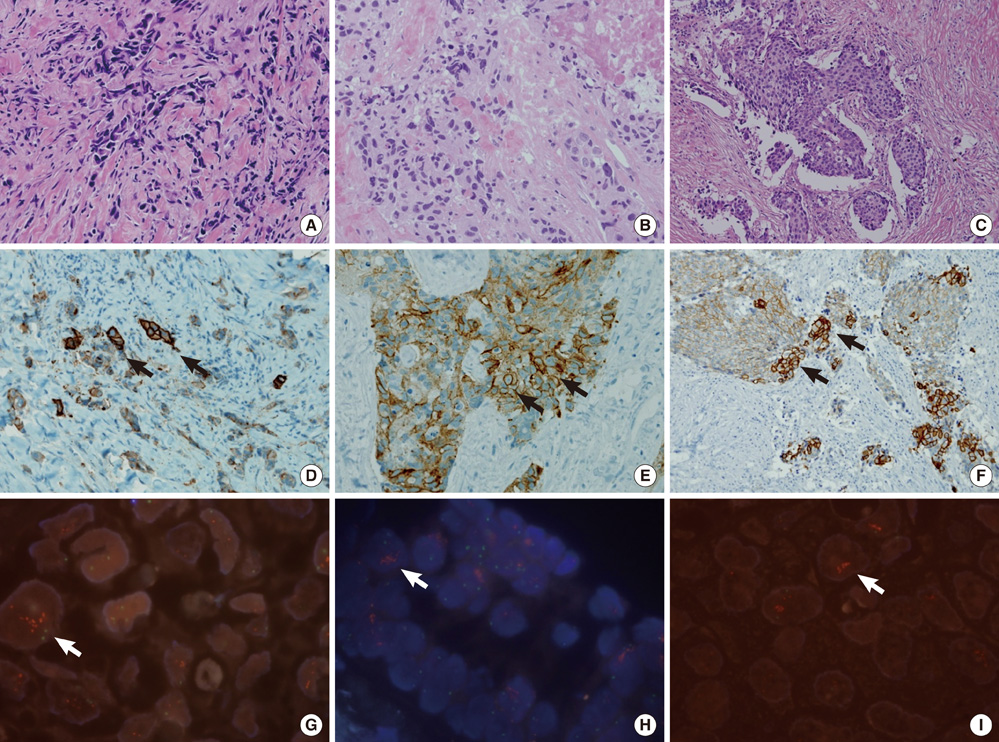
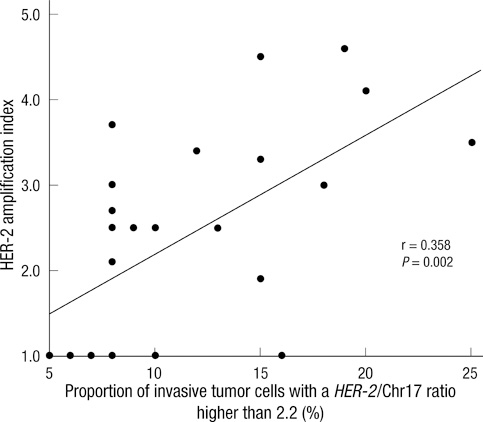
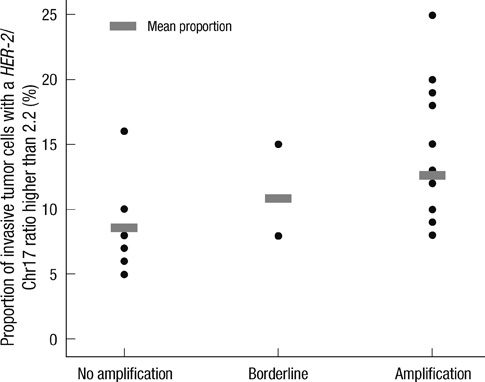
- This study aimed to determine the incidence and characteristics of HER-2 gene heterogeneity in invasive breast cancer in a single institution. Included were 971 cases of primary invasive breast cancer diagnosed between 2008 and 2010. Fluorescence in situ hybridization (FISH) image files were retrospectively reviewed and HER-2 gene heterogeneity was defined as more than 5% but less than 50% of analyzed invasive tumor cells with a HER-2/Chr17 ratio higher than 2.2, according to the College of American Pathologists guidelines. HER-2 gene heterogeneity was identified in 24 (2.5%) cases. The mean proportion of invasive tumor cells with a HER-2/chromosome 17 ratio higher than 2.2 was 11.6% (range: 5%-25%). Of 24 cases, HER-2 gene status was not amplified in 8, showed borderline amplification in 2, and amplification in 14. All HER-2 amplification cases were low-grade. In conclusion, HER-2 gene heterogeneity of invasive breast cancer is identified in routine FISH examination. This may affect the results of HER-2 gene amplification status in FISH studies.
MeSH Terms
Figure
Reference
-
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.2. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989. 244:707–712.3. Borg A, Baldetorp B, Fernö M, Killander D, Olsson H, Sigurdsson H. ERBB2 amplification in breast cancer with a high rate of proliferation. Oncogene. 1991. 6:137–143.4. Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991. 49:650–655.5. Lovekin C, Ellis IO, Locker A, Robertson JF, Bell J, Nicholson R, Gullick WJ, Elston CW, Blamey RW. c-erbB-2 oncoprotein expression in primary and advanced breast cancer. Br J Cancer. 1991. 63:439–443.6. McCann AH, Dervan PA, O'Regan M, Codd MB, Gullick WJ, Tobin BM, Carney DN. Prognostic significance of c-erbB-2 and estrogen receptor status in human breast cancer. Cancer Res. 1991. 51:3296–3303.7. Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Säve-Söderborgh J, Anbazhagan R, Styles J, Rudenstam CM, Golouh R, Reed R. International (Ludwig) Breast Cancer Study Group. Prognostic importance of c-erbB-2 expression in breast cancer. J Clin Oncol. 1992. 10:1049–1056.8. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.9. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005. 353:1659–1672.10. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.11. Pauletti G, Godolphin W, Press MF, Slamon DJ. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene. 1996. 13:63–72.12. Bartlett JM, Going JJ, Mallon EA, Watters AD, Reeves JR, Stanton P, Richmond J, Donald B, Ferrier R, Cooke TG. Evaluating HER2 amplification and overexpression in breast cancer. J Pathol. 2001. 195:422–428.13. Bilous M, Dowsett M, Hanna W, Isola J, Lebeau A, Moreno A, Penault-Llorca F, Rüschoff J, Tomasic G, van de Vijver M. Current perspectives on HER2 testing: a review of national testing guidelines. Mod Pathol. 2003. 16:173–182.14. Kuukasjärvi T, Karhu R, Tanner M, Kahkönen M, Schäffer A, Nupponen N, Pennanen S, Kallioniemi A, Kallioniemi OP, Isola J. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997. 57:1597–1604.15. Aubele M, Mattis A, Zitzelsberger H, Walch A, Kremer M, Hutzler P, Höfler H, Werner M. Intratumoral heterogeneity in breast carcinoma revealed by laser-microdissection and comparative genomic hybridization. Cancer Genet Cytogenet. 1999. 110:94–102.16. Pertschuk LP, Axiotis CA, Feldman JG, Kim YD, Karavattayhayyil SJ, Braithwaite L. Marked intratumoral heterogeneity of the proto-oncogene Her-2/neu determined by three different detection systems. Breast J. 1999. 5:369–374.17. Glöckner S, Buurman H, Kleeberger W, Lehmann U, Kreipe H. Marked intratumoral heterogeneity of c-myc and cyclinD1 but not of c-erbB2 amplification in breast cancer. Lab Invest. 2002. 82:1419–1426.18. Andersson J, Linderholm B, Bergh J, Elmberger G. HER-2/neu (c-erbB-2) evaluation in primary breast carcinoma by fluorescent in situ hybridization and immunohistochemistry with special focus on intratumor heterogeneity and comparison of invasive and in situ components. Appl Immunohistochem Mol Morphol. 2004. 12:14–20.19. Shin SJ, Hyjek E, Early E, Knowles DM. Intratumoral heterogeneity of her-2/neu in invasive mammary carcinomas using fluorescence in-situ hybridization and tissue microarray. Int J Surg Pathol. 2006. 14:279–284.20. Hanna W, Nofech-Mozes S, Kahn HJ. Intratumoral heterogeneity of HER2/neu in breast cancer: a rare event. Breast J. 2007. 13:122–129.21. Brunelli M, Manfrin E, Martignoni G, Miller K, Remo A, Reghellin D, Bersani S, Gobbo S, Eccher A, Chilosi M, Bonetti F. Genotypic intratumoral heterogeneity in breast carcinoma with HER2/neu amplification: evaluation according to ASCO/CAP criteria. Am J Clin Pathol. 2009. 131:678–682.22. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.23. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.24. Tubbs RR, Hicks DG, Cook J, Downs-Kelly E, Pettay J, Hartke MB, Hood L, Neelon R, Myles J, Budd GT, Moore HC, Andresen S, Crowe JP. Fluorescence in situ hybridization (FISH) as primary methodology for the assessment of HER2 status in adenocarcinoma of the breast: a single institution experience. Diagn Mol Pathol. 2007. 16:207–210.25. Ross JS, Fletcher JA. HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999. 112:1 Suppl 1. S53–S67.26. Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ, Isola J. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000. 157:1467–1472.27. Vance GH, Barry TS, Bloom KJ, Fitzgibbons PL, Hicks DG, Jenkins RB, Persons DL, Tubbs RR, Hammond ME. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med. 2009. 133:611–612.28. Nowell PC. The clonal evolution of tumor cell populations. Science. 1976. 194:23–28.29. Shen CY, Yu JC, Lo YL, Kuo CH, Yue CT, Jou YS, Huang CS, Lung JC, Wu CW. Genome-wide search for loss of heterozygosity using laser capture microdissected tissue of breast carcinoma: an implication for mutator phenotype and breast cancer pathogenesis. Cancer Res. 2000. 60:3884–3892.30. Szöllösi J, Balázs M, Feuerstein BG, Benz CC, Waldman FM. ERBB-2 (HER2/neu) gene copy number, p185HER-2 overexpression, and intratumor heterogeneity in human breast cancer. Cancer Res. 1995. 55:5400–5407.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HER2 Status in Gastric Adenocarcinomas Assessed by Immunohistochemistry, Automated Silver-Enhanced In Situ Hybridization and Fluorescence In Situ Hybridization
- Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer
- Automated Silver-enhanced In Situ Hybridization for Evaluation of HER2 Gene Status in Breast Carcinoma: Comparison with Fluorescence In Situ Hybridization and Immunohistochemistry
- A Single Institute's Experience of Standardization for the HER2 Status by Fluorescence in situ Hybridization and Immunohistochemistry on a Primary Breast Cancer Tissue Microarray
- Comparison of Silver-Enhanced in situ Hybridization and Fluorescence in situ Hybridization for HER2 Gene Status in Breast Carcinomas