J Korean Med Sci.
2013 Nov;28(11):1609-1614. 10.3346/jkms.2013.28.11.1609.
A Prospective, Randomized Comparison of Promus Everolimus-Eluting and TAXUS Liberte Paclitaxel-Eluting Stent Systems in Patients with Coronary Artery Disease Eligible for Percutaneous Coronary Intervention: The PROMISE Study
- Affiliations
-
- 1Division of Cardiology, Yeungnam University Medical Center, Daegu, Korea. yjkim@med.yu.ac.kr
- 2Division of Cardiology, Chonnam National University, Gwangju, Korea.
- 3Division of Cardiology, Chungbuk National University Hospital, Cheongju, Korea.
- 4Division of Cardiology, Kunyang University Hospital, Daejeon, Korea.
- 5Division of Cardiology, Chungnam National University Hospital, Daejeon, Korea.
- 6Division of Cardiology, Dankuk Unversity Hospital, Cheonan, Korea.
- 7Division of Cardiology, Eulji University Hospital, Daejeon, Korea.
- 8Division of Cardiology, Wonkwang University Hospital, Iksan, Korea.
- 9Division of Cardiology, Gwangju Christian Hospital, Gwangju, Korea.
- 10Division of Cardiology, St. Carollo Hospital, Sooncheon, Korea.
- 11Division of Cardiology, Kwangju Veterans Hospital, Gwangju, Korea.
- 12Division of Cardiology, Keimyung Univerisity Dongsan Hospital, Daegu, Korea.
- 13Division of Cardiology, Daegu Catholic University Hospital, Daegu, Korea.
- 14Division of Cardiology, Kyungpook National University Hospital, Daegu, Korea.
- 15Division of Cardiology, Donga University Hospital, Busan, Korea.
- 16Division of Cardiology, Kyungsang National University Hospital, Jinju, Korea.
- 17Division of Cardiology, Inje University Haeundae Paik Hospital, Busan, Korea.
- 18Division of Cardiology, Maryknoll Medical Center, Busan, Korea.
- KMID: 1777657
- DOI: http://doi.org/10.3346/jkms.2013.28.11.1609
Abstract
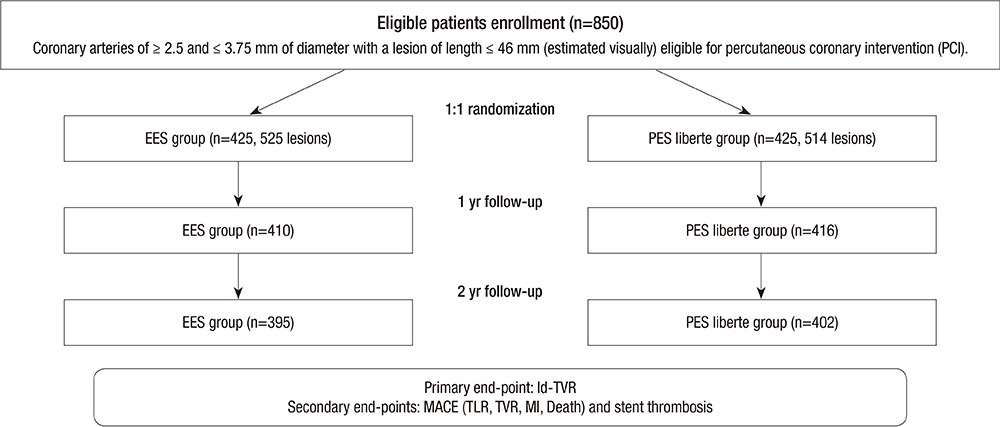
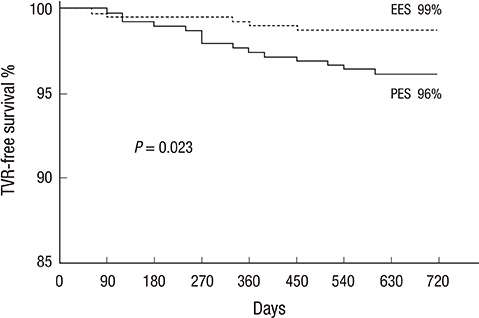
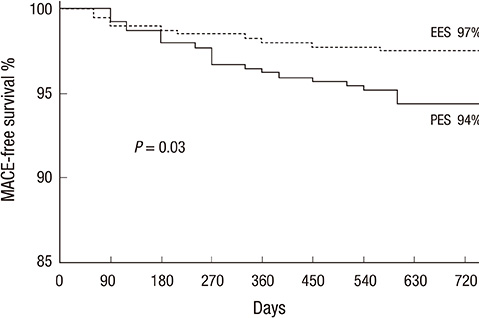
- We aimed comparing two-year clinical outcomes of the Everolimus-Eluting Promus and Paclitaxel-Eluting TAXUS Liberte stents used in routine clinical practice. Patients with objective evidence of ischemia and coronary artery disease eligible for PCI were prospectively randomized to everolimus-eluting stent (EES) or paclitaxel-eluting stent (PES) groups. The primary end-point was ischemia-driven target vessel revascularization (TVR) at 2 yr after intervention, and the secondary end-point was a major adverse cardiac event (MACE), such as death, myocardial infarction (MI), target lesion revascularization (TLR), TVR or stent thrombosis. A total of 850 patients with 1,039 lesions was randomized to the EES (n=425) and PES (n=425) groups. Ischemic-driven TVR at 2 yr was 3.8% in the PES and 1.2% in the EES group (P for non-inferiority=0.021). MACE rates were significantly different; 5.6% in PES and 2.5% in EES (P = 0.027). Rates of MI (0.8% in PES vs 0.2% in EES, P = 0.308), all deaths (1.5% in PES vs 1.2% in EES, P = 0.739) and stent thrombosis (0.3% in PES vs 0.7% in EES, P = 0.325) were similar. The clinical outcomes of EES are superior to PES, mainly due to a reduction in the rate of ischemia-driven TVR.
MeSH Terms
-
Antineoplastic Agents, Phytogenic/administration & dosage/therapeutic use
Coronary Artery Disease/*drug therapy/mortality
Coronary Restenosis/prevention & control
*Drug-Eluting Stents
Female
Humans
Immunosuppressive Agents/administration & dosage/therapeutic use
Male
Middle Aged
Paclitaxel/administration & dosage/*therapeutic use
Percutaneous Coronary Intervention/*methods
Prospective Studies
Sirolimus/administration & dosage/*analogs & derivatives/therapeutic use
Thrombosis
Treatment Outcome
Antineoplastic Agents, Phytogenic
Immunosuppressive Agents
Paclitaxel
Sirolimus
Figure
Reference
-
1. Popma JJ, Leon MB, Moses JW, Holmes DR Jr, Cox N, Fitzpatrick M, Douglas J, Lambert C, Mooney M, Yakubov S, et al. Quantitative assessment of angiographic restenosis after sirolimus-eluting stent implantation in native coronary arteries. Circulation. 2004; 110:3773–3780.2. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004; 350:221–231.3. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351.4. Turco MA, Ormiston JA, Popma JJ, Mandinov L, O'Shaughnessy CD, Mann T, McGarry TF, Wu CJ, Chan C, Webster MW, et al. Polymer-based, paclitaxel-eluting TAXUS Liberté stent in de novo lesions: the pivotal TAXUS ATLAS trial. J Am Coll Cardiol. 2007; 49:1676–1683.5. Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, Zeiher A, Grube E, Haase J, Thuesen L, et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial. EuroIntervention. 2005; 1:58–65.6. Serruys PW, Ruygrok P, Neuzner J, Piek JJ, Seth A, Schofer JJ, Richardt G, Wiemer M, Carrié D, Thuesen L, et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention. 2006; 2:286–294.7. Kedhi E, Gomes ME, Lagerqvist B, Smith JG, Omerovic E, James S, Harnek J, Olivecrona GK. Clinical impact of second-generation everolimus-eluting stent compared with first-generation drug-eluting stents in diabetes mellitus patients: insights from a nationwide coronary intervention register. JACC Cardiovasc Interv. 2012; 5:1141–1149.8. Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, Smits PC. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010; 375:201–209.9. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010; 362:1663–1674.10. Waksman R, Ghali M, Goodroe R, Ryan T, Turco M, Ring M, McGarry T, Dobies D, Shammas N, Steinberg DH, et al. Percutaneous coronary intervention with second-generation paclitaxel-eluting stents versus everolimus-eluting stents in United States contemporary practice (REWARDS TLX Trial). Am J Cardiol. 2012; 110:1119–1124.11. Maluenda G, Mitulescu L, Ben-Dor I, A Gaglia M Jr, Weissman G, Torguson R, F Satler L, Pichard AD, Bernardo NL, Waksman R. Retroperitoneal hemorrhage after percutaneous coronary intervention in the current practice era: clinical outcomes and prognostic value of abdominal/pelvic computed tomography. Catheter Cardiovasc Interv. 2012; 80:29–36.12. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Mahaffey KW, Cutlip DE, Fitzgerald PJ, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008; 299:1903–1913.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in Percutaneous Coronary Intervention in Coronary Artery Disease
- Diffuse Long Coronary Artery Disease is Still an Obstacle for Percutaneous Coronary Intervention in the Second-Generation Drug-Eluting Stent Era?
- Dark Side of Drug-eluting Stent in Contemporary Percutaneous Coronary Intervention
- Novel Coronary Stent Platforms
- Drug-Eluting Stent: Present and Future