Korean J Radiol.
2005 Dec;6(4):241-247. 10.3348/kjr.2005.6.4.241.
Efficacy of a Dexamethasone-Eluting Nitinol Stent on the Inhibition of Pseudointimal Hyperplasia in a Transjugular Intrahepatic Portosystemic Shunt: An Experimental Study in a Swine Model
- Affiliations
-
- 1Department of Diagnostic Radiology, Korea University Guro Hospital, Seoul, Korea.
- 2Department of Diagnostic Radiology, Kyung Hee University Hospital, Seoul, Korea. ohjh6108@hanmail.net
- 3Department of Pathology, Kyung Hee University Hospital, Seoul, Korea.
- 4Department of Radiology, University of Ulsan, College of Medicine, Seoul, Korea.
- 5Department of Diagnostic Radiology, Hallym University, College of Medicine, Seoul, Korea.
- 6Department of Polymer Science and Engineering, Hannam University, Taejon, Korea.
- KMID: 1777282
- DOI: http://doi.org/10.3348/kjr.2005.6.4.241
Abstract
OBJECTIVE
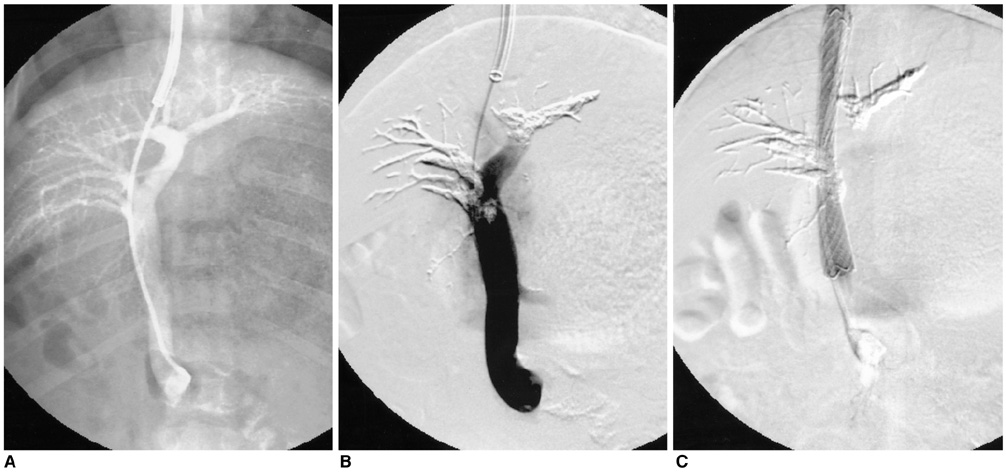
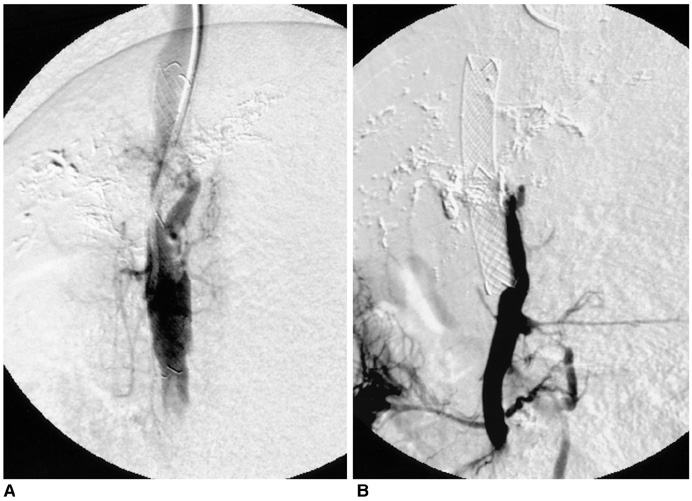
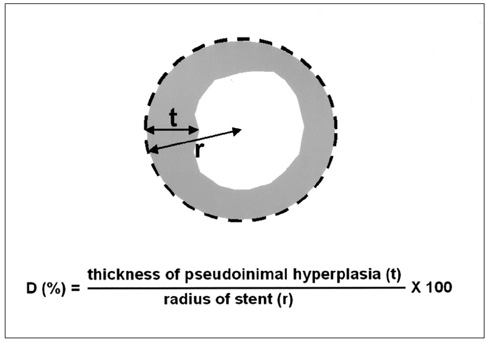
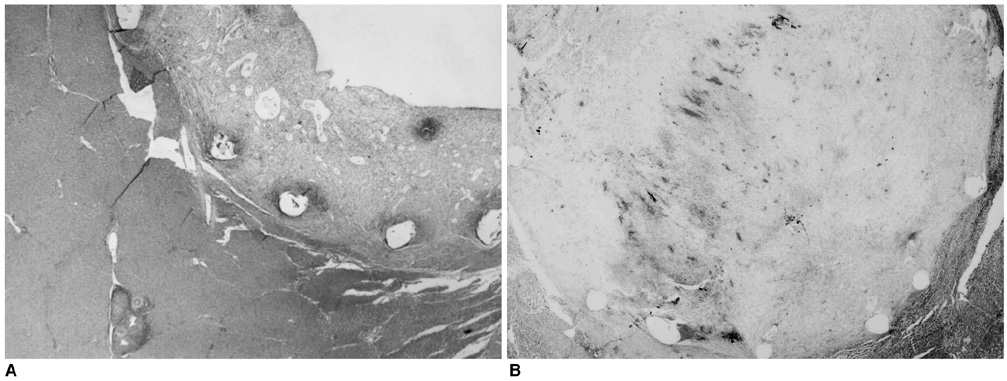
We wanted to evaluate the feasibility and efficacy of using a dexamethasone (DM) -eluting nitinol stent to inhibit the pseudointimal hyperplasia following stent placement in the transjugular intrahepatic portosystemic shunt tract (TIPS) of a swine. MATERIALS AND METHODS: Fifteen stents were constructed using 0.15 mm-thick nitinol wire; they were 60 mm in length and 10 mm in diameter. The metallic stents were then classified into three types; type 1 and 2 was coated with the mixture of 12% and 20%, respectively, of DM solution and polyurethane (PU), while type 3 was a bare stent that was used for control study. In fifteen swine, each type of stent was implanted in the TIPS tract of 5 swine, and each animal was sacrificed 2 weeks after TIPS creation. The proliferation of the pseudointima was evaluated both on follow-up portogram and pathologic examination. RESULTS: One TIPS case, using the type 1 stent, and two TIPS cases, using the type 2 stent, maintained their luminal patency while the others were all occluded. On the histopathologic analysis, the mean of the maximum pseudointimal hyperplasia was expressed as the percentage of the stent radius that was patent, and these values were 51.2%, 50% and 76% for the type 1, 2, and 3 stents, respectively. CONCLUSION: The DM-eluting stent showed a tendency to reduce the development of pseudointimal hyperplasia in the TIPS tract of a swine model with induced-portal hypertension.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhuang ZW, Bettmann MA, Teng GJ, Jeffery RF, Langdon DR. Long-term results in patients with TIPS: an outcome study. J Vasc Interv Radiol. 2001. 12:suppl. S9–S10.2. Haskal ZJ, Pentecost MJ, Soulen MC, Shlansky-Goldberg RD, Baum RA, Cope C. Transjugular intrahepatic portosystemic shunt stenosis and revision: early and midterm results. AJR Am J Roentgenol. 1994. 163:439–444.3. Saxon RS, Ross PL, Mendel-Hartvig J, Barton RE, Benner K, Flora K, et al. Transjugular intrahepatic portosystemic shunt patency and the importance of stenosis location in the development of recurrent symptoms. Radiology. 1998. 207:683–693.4. Duda SH, Poerner TC, Wiesinger B, Rundback JH, Tepe G, Wiskirchen J, et al. Drug-eluting stents: potential applications for peripheral arterial occlusive disease. J Vasc Interv Radiol. 2003. 14:291–301.5. Lincoff AM, Frust JG, Ellis SG, Tuch RJ, Topol EJ. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol. 1997. 29:808–816.6. Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens J, et al. Study of antirestenosis with the BiodivYsio dexamethasoneeluting stent (STRIDE): a first-in-human multicenter pilot trial. Catheter Cardiovasc Interv. 2003. 60:172–178.7. Haskal ZJ, Davis A, McAllister A, Furth EE. PTFE-encapsulated endovascular stent-graft for transjugular intrahepatic portosystemic shunts: experimental evaluation. Radiology. 1997. 205:682–688.8. Kichikawa K, Saxon RR, Nishimine K, Nishida N, Uchida BT. Experimental TIPS with spiral Z-stents in swine with and without induced portal hypertension. Cardiovasc Intervent Radiol. 1997. 20:197–203.9. Teng GJ, Bettmann MA, Hoopes PJ, Wagner RJ, Park BH, Yang L, et al. Transjugular intrahepatic portosystemic shunt: effect of bile leak on smooth muscle cell proliferation. Radiology. 1998. 208:799–805.10. LaBerge JM, Ferrell LD, Ring EJ, Gordon RL. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993. 4:779–786.11. Palmaz JC, Sibbitt RR, Reuter SR, Garcia F, Tio FO. Expandable intrahepatic portacaval shunt stents: early experience in the dog. AJR Am J Roentgenol. 1985. 145:821–825.12. Terayama N, Matsui O, Kadoya M, Yoshikawa J, Gabata T, Miyayama S, et al. Transjugular intrahepatic portosystemic shunt: histologic and immunohistochemical study of autopsy cases. Cardiovasc Intervent Radiol. 1997. 20:457–461.13. Haskal ZJ, Brennecke LJ. Porous and nonporous polycarbonate urethane stent-grafts for TIPS formation: biologic responses. J Vasc Interv Radiol. 1999. 10:1255–1263.14. Haskal ZJ, Brennecke LH. Transjugular intrahepatic portosystemic shunts formed with polyethylene terephthalate-covered stents: experimental evaluation in pigs. Radiology. 1999. 213:853–859.15. Nishimine K, Saxon RR, Kichikawa K, Mendel-Hartvig J, Timmermans HA, Shim HJ, et al. Improved transjugular intrahepatic portosystemic shunt patency with PTFE-covered stent-grafts: experimental results in swine. Radiology. 1995. 196:341–347.16. Otal P, Rousseau H, Vinel JP, Ducoin H, Hassissene S, Joffre F. High occlusion rate in experimental transjugular intrahepatic portosystemic shunt created with a Dacron-covered nitinol stent. J Vasc Interv Radiol. 1999. 10:183–188.17. Tanihata H, Saxon RR, Kubota Y, Pavcnik D, Uchida BT, Rosch J, et al. Transjugular intrahepatic portosystemic shunt with silicone-covered Wallstents: results in a swine model. Radiology. 1997. 205:181–184.18. Lessie T, Yoon HC, Nelson HA, Fillmore DJ, Baldwin GN, Miller FJ. Intraluminal irradiation for TIPS stenosis: preliminary results in a swine model. J Vasc Interv Radiol. 1999. 10:899–906.19. Oseas RS, Allen J, Yang HH, Baehner RL, Boxer LA. Mechanism of dexamethasone inhibition of chemotactic factor induced granulocyte aggregation. Blood. 1982. 59:265–269.20. Chervu A, Moore WS, Quinones-Baldrich WJ, Henderson T. Efficacy of corticosteroids in suppression of intimal hyperplasia. J Vasc Surg. 1989. 10:192–134.21. Colburn MD, Moore WS, Gelabert HA, Quinones-Baldrich WJ. Dose responsive suppression of myointimal hyperplasia by dexamethasone. J Vasc Surg. 1992. 15:510–518.22. Voisard R, Seitzer U, Baur R, Dartsch PC, Osterhues H, Hoher M, et al. Corticosteroid agents inhibit proliferation of smooth muscle cells from human atherosclerotic arteries in vitro. Int J Cardiol. 1994. 43:257–267.23. Stone GW, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, Ligon RW, et al. A randomized trial of corticosteroids for the prevention of restenosis in 102 patients undergoing repeat coronary angioplasty. Cathet Cardiovasc Diagn. 1989. 18:227–231.24. Pepine CJ, Hirshfeld JW, Macdonald RG, Henderson MA, Bass TA, Goldberg S, et al. A controlled trial of corticosteroids to prevent restenosis after coronary angioplasty. M-HEART Group. Circulation. 1990. 81:1753–1176.25. Strecker EP, Gabelmann A, Boos I, Lucas C, Xu Z, Haberstroh J, et al. Effect on intimal hyperplasia of dexamethasone released from coated metal stents compared with non-coated stents in canine femoral arteries. Cardiovasc Intervent Radiol. 1998. 21:487–496.26. Muller DW, Golomb G, Gordon D, Levy RJ. Site-specific dexamethasone delivery for the prevention of neointimal thickening after vascular stent implantation. Coron Artery Dis. 1994. 5:435–442.27. Yoon HK, Park KS, Kang SG, Park SS, Kim TH, Sung KB, et al. Influence of dexamethasone-coated Nitinol stent on neointimal formation in the canine great vessel model. J Korean Radiol Soc. 2001. 44:433–440.28. Jalan R, Harrison DJ, Redhead DN, Hayes PC. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) occlusion and the role of biliary venous fistulae. J Hepatol. 1996. 24:169–176.29. Saxon RR, Mendel-Hartvig J, Corless CL, Rabkin J, Uchida BT, Nishimine K, et al. Bile duct injury as a major cause of stenosis and occlusion in transjugular intrahepatic portosystemic shunts: comparative histopathologic analysis in humans and swine. J Vasc Interv Radiol. 1996. 7:487–497.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of a Paclitaxel-Eluting Nitinol Stent on the Inhibition of Pseudointimal Hyperplasia in a Transjugular Intrahepatic Portosystemic Shunt: An Experimental Study in a Swine Model
- Percutaneous retrieval of a misplaced transjugular intrahepatic portosystemic shunt stent using the rigid endobronchial forceps
- Treating systemic inflammation by transjugular intrahepatic portosystemic shunt: Editorial on “Insertion of a transjugular intrahepatic portosystemic shunt leads to sustained reversal of systemic inflammation in patients with decompensated liver cirrhosis”
- A new and improved transjugular intrahepatic portosystemic shunt (TIPS) stent graft: Controlled expansion
- Recanalization of an Occluded Intrahepatic Portosystemic Covered Stent via the Percutaneous Transhepatic Approach