Yonsei Med J.
2012 May;53(3):593-602. 10.3349/ymj.2012.53.3.593.
Endotoxin Is Not Essential for the Development of Cockroach Induced Allergic Airway Inflammation
- Affiliations
-
- 1Department of Internal Medicine, Institute of Allergy, Yonsei University College of Medicine, Seou, Korea. parkjw@yuhs.ac
- 2Department of Life Science, Biomedical Research Institute, Hanyang University, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pediatrics, University of Ulsan College of Medicine, Seoul, Korea.
- 5Center for Immunology and Pathology, Korea National Institute of Health, Cheongwon, Korea.
- KMID: 1776996
- DOI: http://doi.org/10.3349/ymj.2012.53.3.593
Abstract
- PURPOSE
Cockroach (CR) is an important inhalant allergen and can induce allergic asthma. However, the mechanism by which CR induces airway allergic inflammation and the role of endotoxin in CR extract are not clearly understood in regards to the development of airway inflammation. In this study, we evaluated whether endotoxin is essential to the development of CR induced airway allergic inflammation in mice.
MATERIALS AND METHODS
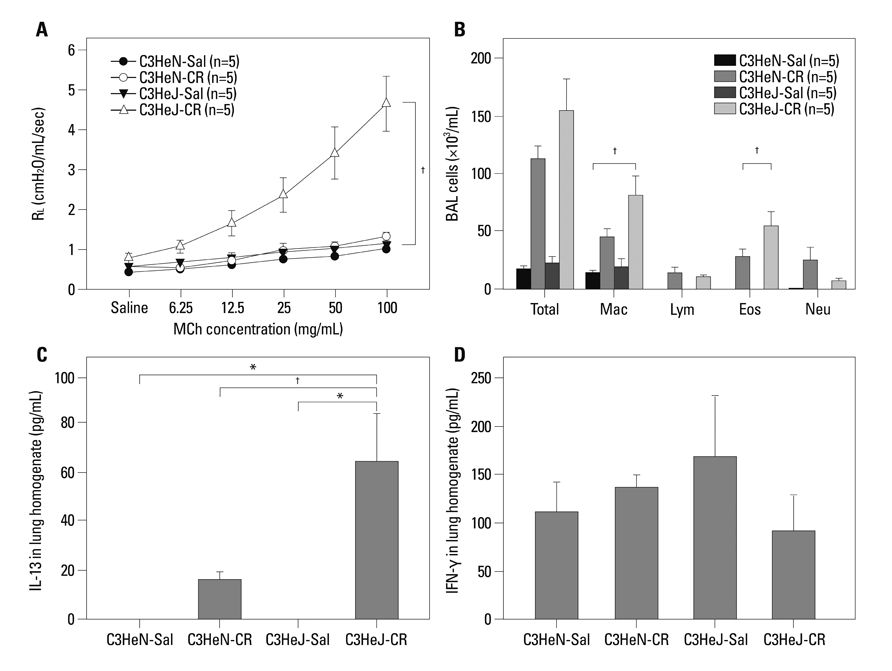
Airway allergic inflammation was induced by intranasal administration of either CR extract, CR with additional endotoxin, or endotoxin depleted CR extract, respectively, in BALB/c wild type mice. CR induced inflammation was also evaluated with toll like receptor-4 (TLR-4) mutant (C3H/HeJ) and wild type (C3H/HeN) mice.
RESULTS
Intranasal administration of CR extracts significantly induced airway hyperresponsiveness (AHR), eosinophilic and neutrophilic airway inflammation, as well as goblet cell hyperplasia in a dose-dependent manner. The addition of endotoxin along with CR allergen attenuated eosinophilic inflammation, interleukin (IL)-13 level, and goblet cell hyperplasia of respiratory epithelium; however, it did not affect the development of AHR. Endotoxin depletion in CR extract did not attenuate eosinophilic inflammation and lymphocytosis in BAL fluid, AHR and IL-13 expression in the lungs compared to CR alone. The attenuation of AHR, eosinophilic inflammation, and goblet cell hyperplasia induced by CR extract alone was not different between TLR-4 mutant and the wild type mice. In addition, heat inactivated CR extract administration induced attenuated AHR and eosinophilic inflammation.
CONCLUSION
Endotoxin in CR extracts may not be essential to the development of airway inflammation.
Keyword
MeSH Terms
-
Allergens/*immunology
Animals
Asthma/*chemically induced/*immunology/metabolism
Cockroaches/*immunology
Endotoxins/*immunology
Enzyme-Linked Immunosorbent Assay
Female
Inflammation/*chemically induced/*immunology/metabolism
Interferon-gamma/metabolism
Interleukin-13/metabolism
Interleukin-5/metabolism
Mice
Mice, Inbred BALB C
Respiratory Hypersensitivity/chemically induced/*immunology
Figure
Cited by 1 articles
-
Protease-Activated Receptors 2-Antagonist Suppresses Asthma by Inhibiting Reactive Oxygen Species-Thymic Stromal Lymphopoietin Inflammation and Epithelial Tight Junction Degradation
Ha-Jung Kim, Seung-Hwa Lee, Sekyoo Jeong, Soo-Jong Hong
Allergy Asthma Immunol Res. 2019;11(4):560-571. doi: 10.4168/aair.2019.11.4.560.
Reference
-
1. Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NF Jr. Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. J Allergy Clin Immunol. 1996. 97:1393–1401.
Article2. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997. 336:1356–1363.
Article3. Busse WW, Mitchell H. Addressing issues of asthma in inner-city children. J Allergy Clin Immunol. 2007. 119:43–49.
Article4. Bice DE, Seagrave J, Green FH. Animal models of asthma: potential usefulness for studying health effects of inhaled particles. Inhal Toxicol. 2000. 12:829–862.
Article5. de Siqueira AL, Russo M, Steil AA, Facincone S, Mariano M, Jancar S. A new murine model of pulmonary eosinophilic hypersensitivity: contribution to experimental asthma. J Allergy Clin Immunol. 1997. 100:383–388.
Article6. Clarke AH, Thomas WR, Rolland JM, Dow C, O'Brien RM. Murine allergic respiratory responses to the major house dust mite allergen Der p 1. Int Arch Allergy Immunol. 1999. 120:126–134.
Article7. Warner RL, Lukacs NW, Shapiro SD, Bhagarvathula N, Nerusu KC, Varani J, et al. Role of metalloelastase in a model of allergic lung responses induced by cockroach allergen. Am J Pathol. 2004. 165:1921–1930.
Article8. Smith H. Animal models of asthma. Pulm Pharmacol. 1989. 2:59–74.
Article9. Arruda LK, Vailes LD, Ferriani VP, Santos AB, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001. 107:419–428.
Article10. Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004. 113:315–319.
Article11. Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J Immunol. 2001. 167:2808–2815.
Article12. Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007. 179:2910–2917.
Article13. Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011. 186:3164–3172.
Article14. Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009. 50:22–30.
Article15. Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011. 52:393–400.
Article16. Hasday JD, Bascom R, Costa JJ, Fitzgerald T, Dubin W. Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999. 115:829–835.
Article17. Reed CE, Milton DK. Endotoxin-stimulated innate immunity: a contributing factor for asthma. J Allergy Clin Immunol. 2001. 108:157–166.
Article18. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002. 347:869–877.
Article19. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000. 55:Suppl 1. S2–S10.
Article20. Matricardi PM, Franzinelli F, Franco A, Caprio G, Murru F, Cioffi D, et al. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol. 1998. 101(4 Pt 1):439–444.
Article21. Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996. 154(6 Pt 1):1641–1646.
Article22. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, et al. Opposite effects of CD 14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005. 116:601–607.
Article23. Zambelli-Weiner A, Ehrlich E, Stockton ML, Grant AV, Zhang S, Levett PN, et al. Evaluation of the CD14/-260 polymorphism and house dust endotoxin exposure in the Barbados Asthma Genetics Study. J Allergy Clin Immunol. 2005. 115:1203–1209.
Article24. Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009. 123:612–618.
Article25. Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998. 161:7047–7053.26. Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy. 2007. 37:1364–1373.
Article27. Russo M, Lutton JD. Decreased in vivo and in vitro colony stimulating activity responses to bacterial lipopolysaccharide in C3H/HeJ mice. J Cell Physiol. 1977. 92:303–307.
Article28. Pritchard DI, Eady RP, Harper ST, Jackson DM, Orr TS, Richards IM, et al. Laboratory infection of primates with Ascaris suum to provide a model of allergic bronchoconstriction. Clin Exp Immunol. 1983. 54:469–476.29. Mapp C, Hartiala J, Frick OL, Shields RL, Gold WM. Airway responsiveness to inhaled antigen, histamine, and methacholine in inbred, ragweed-sensitized dogs. Am Rev Respir Dis. 1985. 132:292–298.30. Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011. 17:479–487.
Article31. Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, et al. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol. 2002. 27:526–535.
Article32. Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol. 1996. 14:425–438.
Article33. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004. 169:378–385.
Article34. Zhou D, Chen G, Kim JT, Lee LY, Kang BC. A dose-response relationship between exposure to cockroach allergens and induction of sensitization in an experimental asthma in Hartley guinea pigs. J Allergy Clin Immunol. 1998. 101:653–659.
Article35. McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. Reproducibility of a novel model of murine asthma-like pulmonary inflammation. Clin Exp Immunol. 2004. 136:224–231.
Article36. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002. 196:1645–1651.
Article37. Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, et al. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005. 115:459–467.
Article38. Wan GH, Li CS, Lin RH. Airborne endotoxin exposure and the development of airway antigen-specific allergic responses. Clin Exp Allergy. 2000. 30:426–432.
Article39. Tulic MK, Holt PG, Sly PD. Modification of acute and late-phase allergic responses to ovalbumin with lipopolysaccharide. Int Arch Allergy Immunol. 2002. 129:119–128.
Article40. Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002. 169:5904–5911.
Article41. Natarajan S, Kim J, Bouchard J, Cruikshank W, Remick DG. Reducing LPS content in cockroach allergens increases pulmonary cytokine production without increasing inflammation: a randomized laboratory study. BMC Pulm Med. 2011. 11:12.
Article42. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001. 1:135–145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intranasal Administration of Unmethylated CpG with Cockroach Antigen Prevents the Development of Cockroach-Induced Allergic Inflammation
- The effects of early allergen/endotoxin exposure on subsequent allergic airway inflammation to allergen in mouse model of asthma
- The Effect of Ascorbic acid on Endotoxin-induced Fibrosis
- Airway epithelial cells in airway inflammation and remodeling in asthma
- dNP2-ctCTLA-4 inhibits German cockroach extract-induced allergic airway inflammation and hyper-responsiveness via inhibition of Th2 responses