Korean J Gastroenterol.
2013 Jan;61(1):30-36. 10.4166/kjg.2013.61.1.30.
Efficacy of Entecavir Switching Therapy in Chronic Hepatitis B Patients with Clevudine-induced Myopathy
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea. kimys@gilhospital.com
- KMID: 1775784
- DOI: http://doi.org/10.4166/kjg.2013.61.1.30
Abstract
- BACKGROUND/AIMS
Clevudine is a potent antiviral agent against HBV. However, long-term clevudine therapy may cause myopathy. This study was carried out to identify the efficacy of entecavir switching therapy in chronic hepatitis B patients experiencing clevudine-induced myopathy.
METHODS
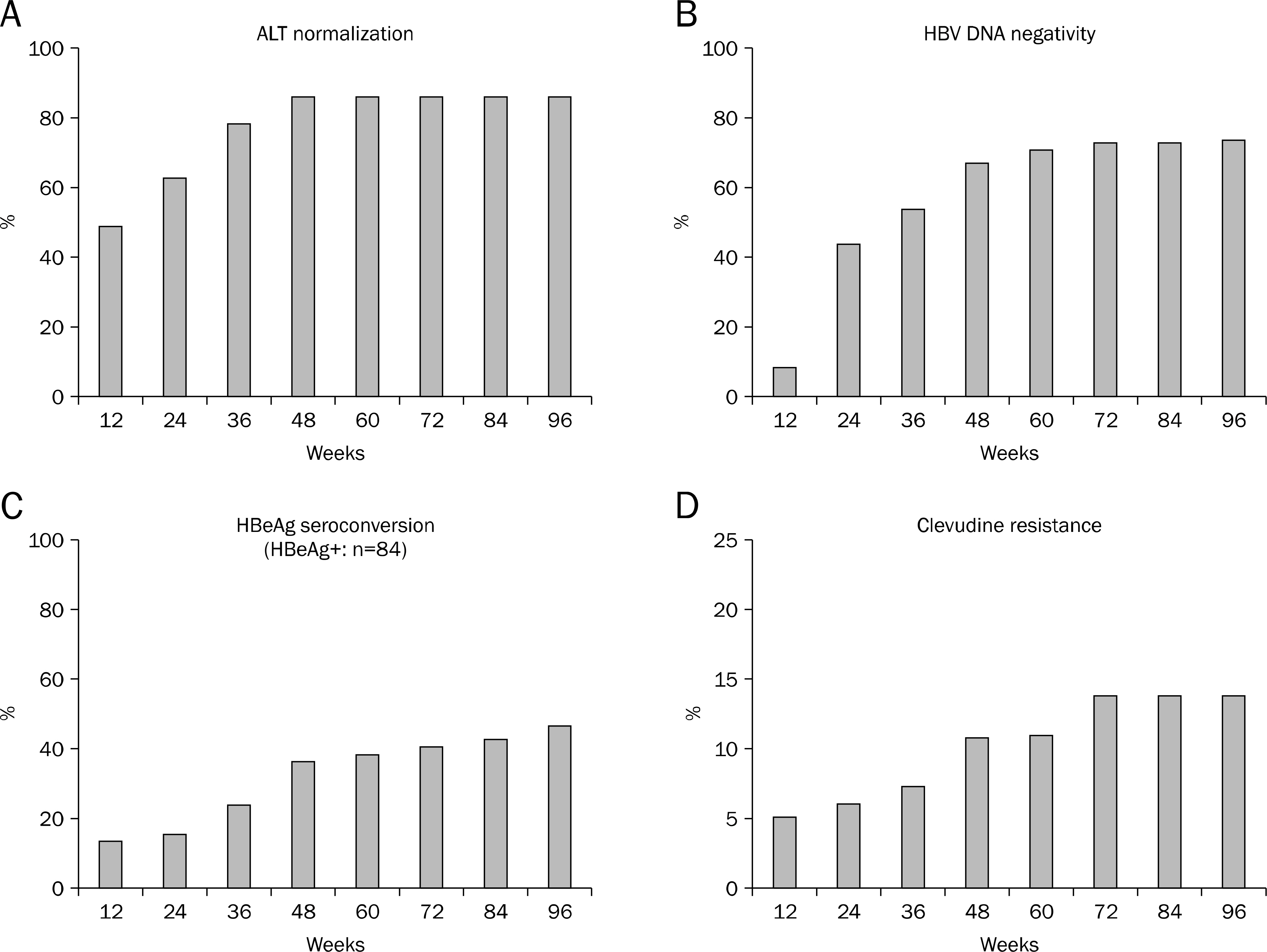
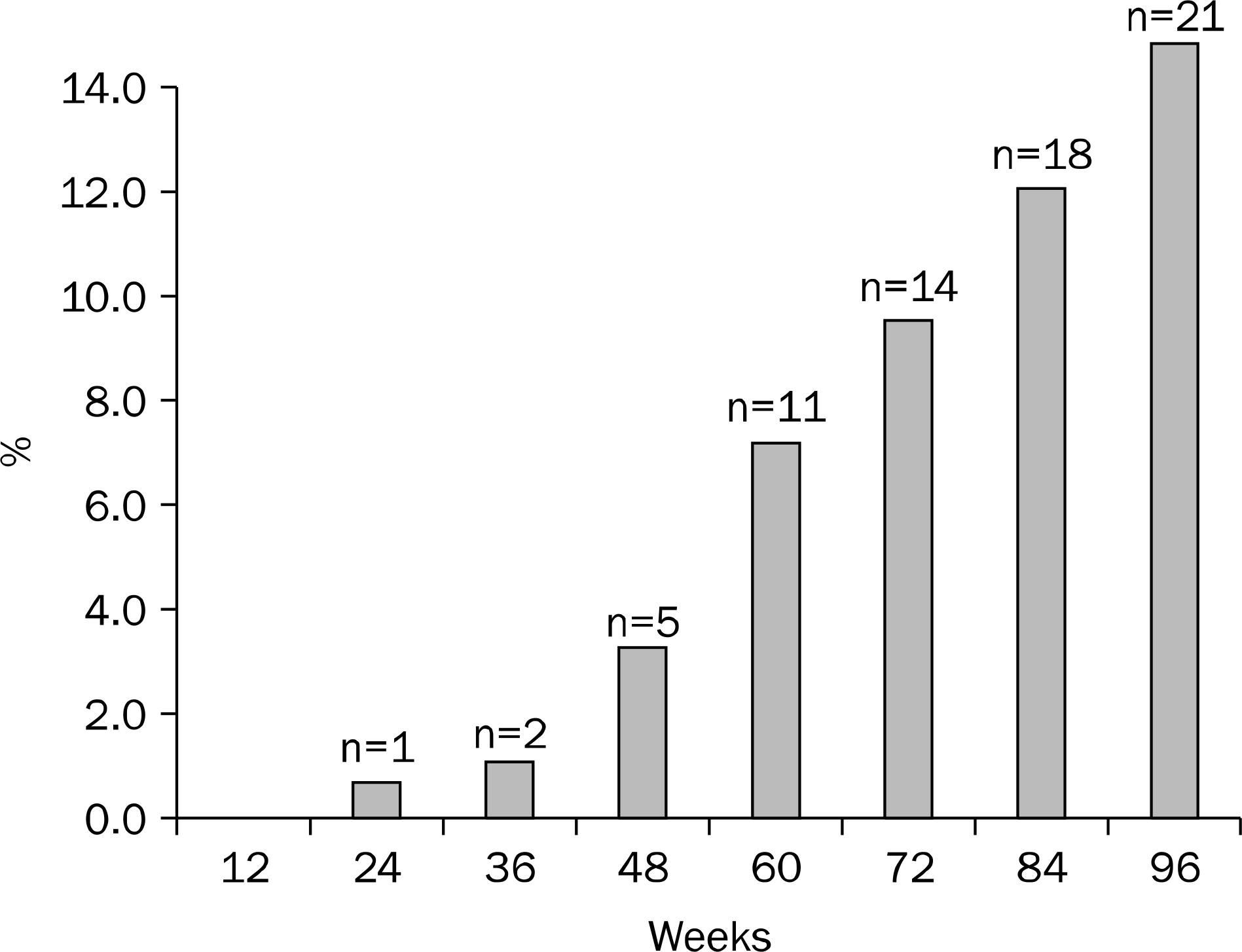
One hundred forty six patients with chronic hepatitis B treated with 30 mg of clevudine per day for 73 weeks (range, 36-132 weeks) were enrolled. Among them, clevudine-induced myopathy occurred in 21 patients (14.4%) which was diagnosed if the patients had symptoms related to myopathy with concurrent CK and AST elevation. All the patients who were diagnosed as clevudine-induced myopathy stopped the therapy, and 17 patients (81%) were switched to entecavir 0.5 mg.
RESULTS
The patients with clevudine-induced myopathy were switched to entecavir 0.5 mg for median 68 weeks, and all of them showed disappearance of clinical myopathic symptoms and normalization of CK and AST level within median 2.2 months. Eight patients (47%) were HBeAg positive before entecavir treatment, and HBeAg seroconversion was achieved in 2 patients (25%). HBV DNA level was elevated in 3 patients (17.6%) at the time when the patients were diagnosed as myopathy, all of them achieved virological response with entecavir switching therapy. ALT level was elevated in 3 patients (17.6%) before entecavir treatment, all of them showed normalization of ALT level. During entecavir therapy, genotypic resistance to entecavir or virological breakthrough was not noted.
CONCLUSIONS
In chronic hepatitis B patients experiencing clevudine-induced myopathy, switching to entecavir 0.5 mg per day showed a resolution of myopathy and adequate viral suppression.
Keyword
MeSH Terms
-
Adult
Aged
Alanine Transaminase/analysis
Antiviral Agents/*adverse effects/therapeutic use
Arabinofuranosyluracil/adverse effects/*analogs & derivatives/therapeutic use
Creatine Kinase/analysis
DNA, Viral/blood
Drug Resistance, Viral
Female
Guanine/*analogs & derivatives/therapeutic use
Hepatitis B e Antigens/blood
Hepatitis B virus/genetics
Hepatitis B, Chronic/*drug therapy
Humans
Male
Middle Aged
Muscular Diseases/*chemically induced
Antiviral Agents
DNA, Viral
Hepatitis B e Antigens
Arabinofuranosyluracil
Guanine
Alanine Transaminase
Creatine Kinase
Figure
Cited by 1 articles
-
Antiviral Effect of Entecavir Switching Therapy in Chronic Hepatitis B Patients with Clevudine-associated Myopathy
Won Young Tak
Korean J Gastroenterol. 2013;61(1):1-2. doi: 10.4166/kjg.2013.61.1.1.
Reference
-
References
1. Shin JW, Park NH. Treatment of chronic hepatitis B. Korean J Med. 2009; 77:265–274.2. Chen CJ, Yang HI, Su J, et al. REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006; 295:65–73.
Article3. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006; 130:678–686.
Article4. Yang HI, Lu SN, Liaw YF, et al. Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002; 347:168–174.
Article5. Korba BE, Furman PA, Otto MJ. Clevudine: a potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Rev Anti Infect Ther. 2006; 4:549–561.6. Lee HJ, Eun JR, Lee CH, et al. Longterm clevudine therapy in nucleos(t)ide-naïve and lamivudine-experienced patients with hepatitis B virus-related chronic liver diseases. Korean J Hepatol. 2009; 15:179–192.
Article7. Shin SR, Yoo BC, Choi MS, et al. A comparison of 48-week treatment efficacy between clevudine and entecavir in treatmentnaïve patients with chronic hepatitis B. Hepatol Int. 2011; 5:664–670.
Article8. Yoo BC, Kim JH, Kim TH, et al. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology. 2007; 46:1041–1048.
Article9. Zhu Y, Yamamoto T, Cullen J, et al. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001; 75:311–322.
Article10. Summers J, Mason WS. Residual integrated viral DNA after hepadnavirus clearance by nucleoside analog therapy. Proc Natl Acad Sci U S A. 2004; 101:638–640.
Article11. Yang HW, Lee BS, Lee TH, et al. Efficacy of initial treatment with clevudine in naive patients with chronic hepatitis B. Korean J Intern Med. 2010; 25:372–376.
Article12. Kim MH, Kim KA, Lee JS, et al. Efficacy of 48-week clevudine therapy for chronic hepatitis B. Korean J Hepatol. 2009; 15:331–337.
Article13. Tak WY, Park SY, Jung MK, et al. Mitochondrial myopathy caused by clevudine therapy in chronic hepatitis B patients. Hepatol Res. 2009; 39:944–947.
Article14. Seok JI, Lee DK, Lee CH, et al. Longterm therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA. Hepatology. 2009; 49:2080–2086.
Article15. Kim BK, Oh J, Kwon SY, et al. Clevudine myopathy in patients with chronic hepatitis B. J Hepatol. 2009; 51:829–834.
Article16. Kim HJ, Park DI, Park JH, et al. Comparison between clevudine and entecavir treatment for antiviral-naíve patients with chronic hepatitis B. Liver Int. 2010; 30:834–840.
Article17. Tak WY, Park SY, Cho CM, et al. Clinical, biochemical, and pathological characteristics of clevudine-associated myopathy. J Hepatol. 2010; 53:261–266.
Article18. Fleischer RD, Lok AS. Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. J Hepatol. 2009; 51:787–791.
Article19. Seifer M, Hamatake RK, Colonno RJ, Standring DN. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob Agents Chemother. 1998; 42:3200–3208.
Article20. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50:661–662.
Article21. Ono SK, Kato N, Shiratori Y, et al. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001; 107:449–455.
Article22. Chang TT, Gish RG, de Man R, et al. BEHoLD AI463022 Study Group. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006; 354:1001–1010.
Article23. Lai CL, Shouval D, Lok AS, et al. BEHoLD AI463027 Study Group. Entecavir versus lamivudine for patients with HBeAgnegative chronic hepatitis B. N Engl J Med. 2006; 354:1011–1020.
Article24. Ko SY, Kwon SY, Choe WH, Kim BK, Kim KH, Lee CH. Clinical and virological responses to clevudine therapy in chronic hepatitis B patients: results at 1 year of an open-labelled prospective study. Antivir Ther. 2009; 14:585–590.25. Liaw YF, Gane E, Leung N, et al. GLOBE Study Group. 2-year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009; 136:486–495.
Article26. Zhang XS, Jin R, Zhang SB, Tao ML. Clinical features of adverse reactions associated with telbivudine. World J Gastroenterol. 2008; 14:3549–3553.
Article27. Heo J, Park JY, Lee HJ, et al. A 96-week randomizaed trial of switching to drug with a higher genetic barrier in chronic hepatitis B patients with partial virologic response to drug with a low genetic barrier. Hepatology. 2011; 54(Suppl 1):1029A–1030A. Poster 1410.28. Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010; 51:422–430.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiviral Effect of Entecavir Switching Therapy in Chronic Hepatitis B Patients with Clevudine-associated Myopathy
- Efficacy of New Anti-viral Agent in the Treatment of Chronic Hepatitis B
- Clevudine therapy in patients with chronic hepatitis B
- Development of Clevudine Resistance after Switching from Lamivudine in a Patient with Chronic Hepatitis B
- Maintaining Antiviral Efficacy after Switching to Generic Entecavir 1 mg for Antiviral-resistant Chronic Hepatitis B