Korean J Radiol.
2008 Jun;9(3):205-211. 10.3348/kjr.2008.9.3.205.
The Steroid Effect on the Blood-Ocular Barrier Change Induced by Triolein Emulsion as seen on Contrast-Enhanced MR Images
- Affiliations
-
- 1Department of Radiology, Graduate School of Inje University, College of Medicine, Pusan, Korea.
- 2Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA.
- 3Department of Radiology & Medical Research Institute & Medical Research Center for Ischemic Tissue Regeneration, Pusan, Korea. hakjink@pusan.ac.kr
- 4Department of Ophthalmology, College of Medicine, Pusan National University, Pusan, Korea.
- 5Department of Preventive Medicine, College of Medicine, Pusan National University, Pusan, Korea.
- 6Department of Parasitology, College of Medicine, Pusan National University, Pusan, Korea.
- KMID: 1758453
- DOI: http://doi.org/10.3348/kjr.2008.9.3.205
Abstract
OBJECTIVE
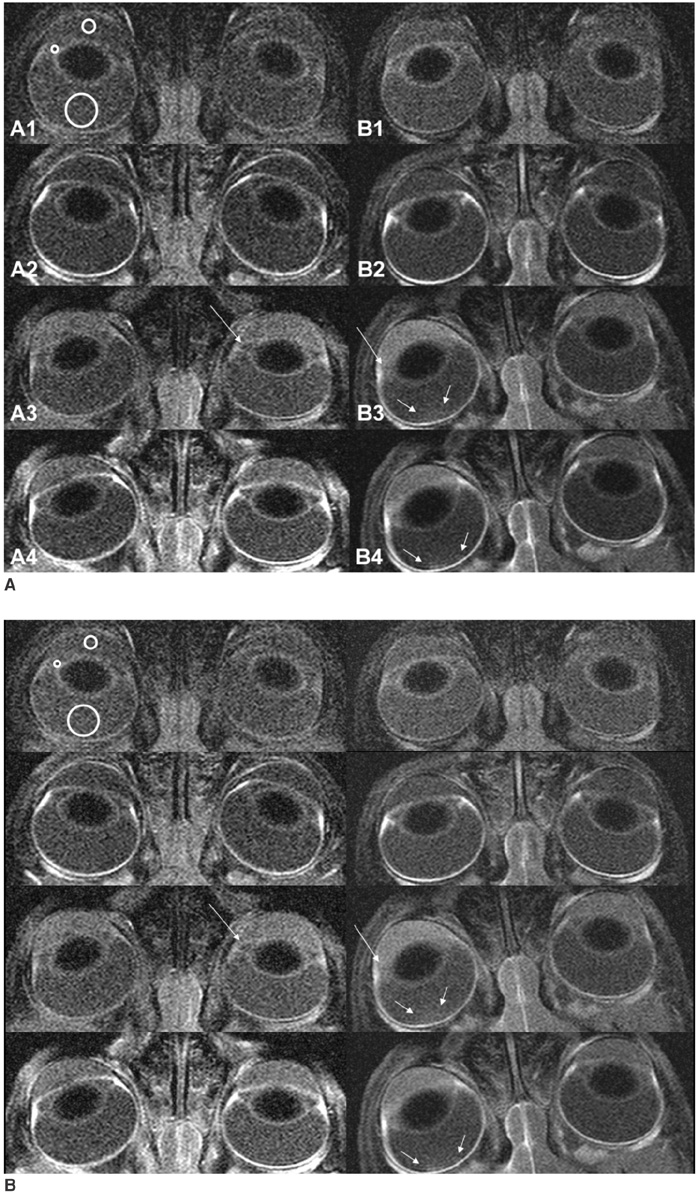
The purpose of this study is to evaluate the effect of dexamethasone on the damaged blood-ocular barrier caused by triolein emulsion, using contrast-enhanced MR imaging. MATERIALS AND METHODS: An emulsion of 0.1-mL triolein in 20 mL of saline was infused into the carotid arteries of 32 cats, 12 cats were placed in the treatment group and 18 cats were placed in the Control group. Thirty minutes after the infusion of triolein emulsion, a set of orbital pre- and post-contrast T1-weighted MR images (T1WIs) were obtained. Infusion of 10 mg/kg dexamethasone into the ipsilateral carotid artery of each of the cats in the treatment group cats and 20 mL saline in each of the cats in the control group was given. A second set of pre- and post-contrast orbital T1WIs were obtained three hours following triolein emulsion infusion. Qualitative analysis was performed for the the anterior chamber (AC), the posterior chamber (PC), and in the vitreous humor of the ipsilateral and contralateral eyes. The signal intensity ratios of the ipsilateral eye over the contralateral eye were quantitatively evaluated in the three ocular chambers on the first and second set of T1WIs, and were then statistically compared. RESULTS: Qualitatively, the AC, the PC or the vitreous did not show immediate contrast enhancement on the first and the second set of post-contrast T1WIs. However, the AC and the PC showed delayed contrast enhancement for both groups of cats on the second pre-contrast T1WIs. No enhancement or minimally delayed enhancement was seen for the vitreous humor. Quantitatively, the signal intensity ratios in the PC of the treatment group of cats were statistically lower than the ratios of the control group of cats for the second set of T1WIs (p = 0.037). The AC and vitreous showed no statistically significant difference between the feline treatment group and control group (p > 0.05). CONCLUSION: Contrast-enhanced MR images revealed increased vascular permeability in the PC of the eye after infusion of triolein emulsion. Dexamethasone seems to decrease the breakdown of the blood-aqueous barrier in the PC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Study of Feasibility of Brain Imaging in Medium- and Small-Sized Animals: Using a Clinical 3T MR System with Three Surface Coils
Shin Young Park, Mi Ri Jeong, Byung Mann Cho, Kang Soo Kim, Hak Jin Kim
J Korean Soc Radiol. 2017;77(5):317-326. doi: 10.3348/jksr.2017.77.5.317.
Reference
-
1. Kim HJ, Lee CH, Kim HG, Lee SD, Son SM, Kim YW, et al. Reversible MR changes in the cat brain after cerebral fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2004. 25:958–963.2. Kim HJ, Lee CH, Lee SH, Moon TY. Magnetic resonance imaging and histologic findings of experimental cerebral fat embolism. Invest Radiol. 2003. 38:625–634.3. Kim HJ, Lee CH, Lee SH, Cho BM, Kim HK, Park BR, et al. Early development of vasogenic edema in experimental cerebral fat embolism in cats: correlation with MRI and electron microscopic findings. Invest Radiol. 2001. 36:460–469.4. Kim HJ, Lee JH, Lee CH, Lee SH, Moon TY, Cho BM, et al. Experimental cerebral fat embolism: embolic effects of triolein and oleic acid depicted by MR imaging and electron microscopy. AJNR Am J Neuroradiol. 2002. 23:1516–1523.5. Boyd HM, Peltier LF, Scott JR, Wheeler DH. Fat embolism. II. The chemical composition of fat obtained from human long bones and subcutaneous tissue. Surgery. 1956. 40:661–664.6. Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979. 23:279–296.7. Pederson JE, Green K. Aqueous humor dynamics: experimental studies. Exp Eye Res. 1973. 15:277–297.8. Verbraeken H, Verstraete A, Van de Velde E, Verschraegen G. Penetration of gentamicin and ofloxacin in human vitreous after systemic administration. Graefes Arch Clin Exp Ophthalmol. 1996. 234:Suppl 1. S59–S65.9. Berkowitz BA, Tofts PS, Sen HA, Ando N, de Juan E Jr. Accurate and precise measurement of blood-retinal barrier breakdown using dynamic Gd-DTPA MRI. Invest Ophthalmol Vis Sci. 1992. 33:3500–3506.10. Kolodny NH, Goode ST, Ryan W, Freddo TF. Evaluation of therapeutic effectiveness using MR imaging in a rabbit model of anterior uveitis. Exp Eye Res. 2002. 74:483–491.11. Severinghaus JW. Hypothetical roles of angiogenesis, osmotic swelling, and ischemia in high-altitude cerebral edema. J Appl Physiol. 1995. 79:375–379.12. Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002. 109:920–927.13. Manfre L, Midiri M, Giuffre G, Mangiameli A, Cardella G, Ponte F, et al. Blood-ocular barrier damage: use of contrast-enhanced MRI. Eur Radiol. 1997. 7:110–114.14. Freddo TF, Patz S, Arshanskiy Y. Pilocarpine's effects on the blood-aqueous barrier of the human eye as assessed by high-resolution, contrast magnetic resonance imaging. Exp Eye Res. 2006. 82:458–464.15. Kolodny NH, Freddo TF, Lawrence BA, Suarez C, Bartels SP. Contrast-enhanced magnetic resonance imaging confirmation of an anterior protein pathway in normal rabbit eyes. Invest Ophthalmol Vis Sci. 1996. 37:1602–1607.16. Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990. 31:125–137.17. Barsotti MF, Bartels SP, Freddo TF, Kamm RD. The source of protein in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci. 1992. 33:581–595.18. Chuang EL, Miller FS 3rd, Kalina RE. Retinal lesions following long bone fractures. Ophthalmology. 1985. 92:370–374.19. Jenkins K, Chung F, Wennberg R, Etchells EE, Davey R. Fat embolism syndrome and elective knee arthroplasty. Can J Anaesth. 2002. 49:19–24.20. Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. 'Low-dose' corticosteroid prophylaxis against fat embolism. J Trauma. 1987. 27:1173–1176.21. Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. A double-blind therapeutic study. J Bone Joint Surg Br. 1987. 69:128–131.22. Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Pathophysiological relevance and possible prognostic value. Lancet. 1980. 1:947–949.23. Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005. 80:249–258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Model for Research on the Blood-Ocular Barrier
- Blood-Brain Barrier Experiments with Clinical Magnetic Resonance Imaging and an Immunohistochemical Study
- Temporary Opening of the Testis-blood Barrier by Triolein Fat Emulsion
- The Effect of Micro-Particles of Linoleic Acid Emulsion on the Blood-Brain Barrier in Cats
- A Study of Feasibility of Brain Imaging in Medium- and Small-Sized Animals: Using a Clinical 3T MR System with Three Surface Coils