Korean J Clin Neurophysiol.
2014 Dec;16(2):62-69. 10.14253/kjcn.2014.16.2.62.
Plasticity Associated Changes in Neurophysiological Tests Following Non Invasive Brain Stimulation in Stroke Rat Model
- Affiliations
-
- 1Department of Rehabilitation Medicine, Chungnam National University School of Medicine, Daejeon, Korea. drjeesungju@cnuh.co.kr
- 2Department of Neurology, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 1737966
- DOI: http://doi.org/10.14253/kjcn.2014.16.2.62
Abstract
- BACKGROUND
Neuromodulation therapy has been used to an adjunctive treatment promoting motor recovery in stroke patients. The objective of the study was to determine the effect of repetitive transcranial magnetic stimulation (rTMS) on neurobehavioral recovery and evoked potentials in rats with middle cerebral artery occlusion.
METHODS
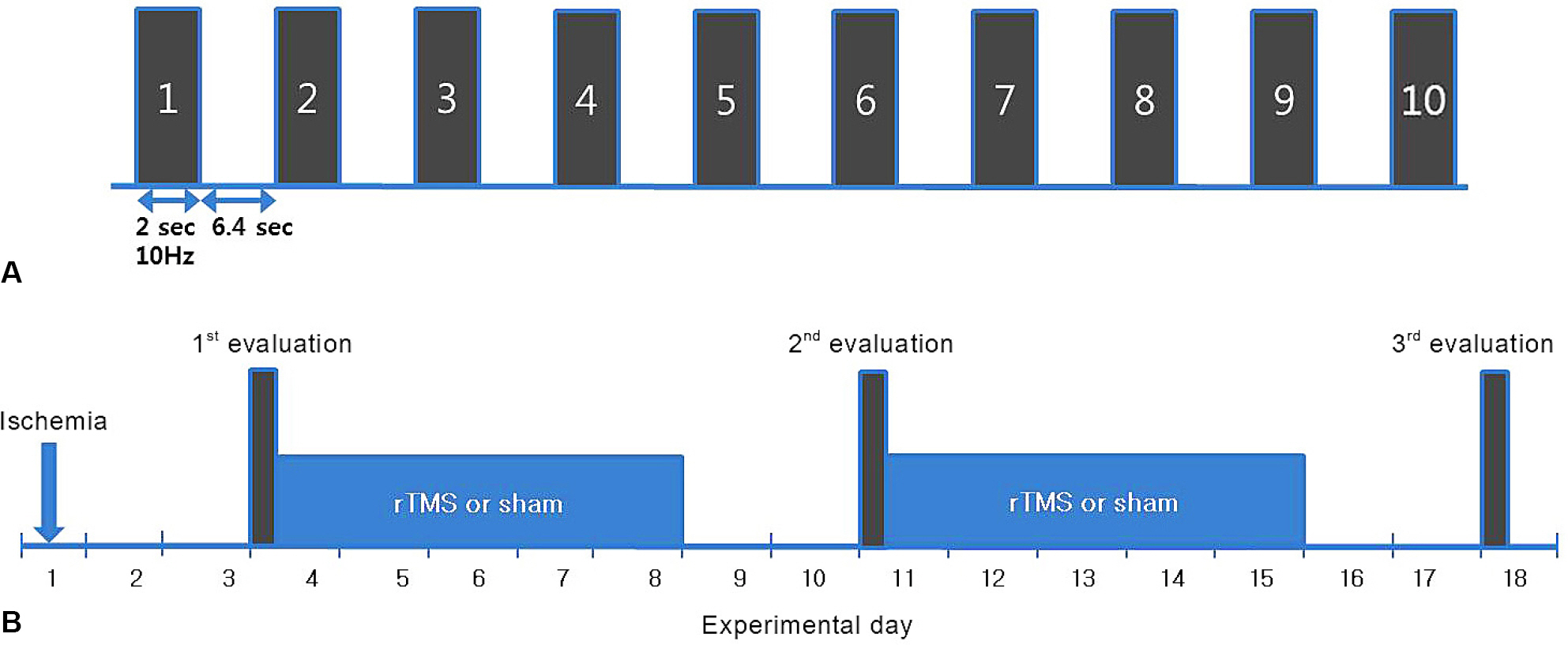
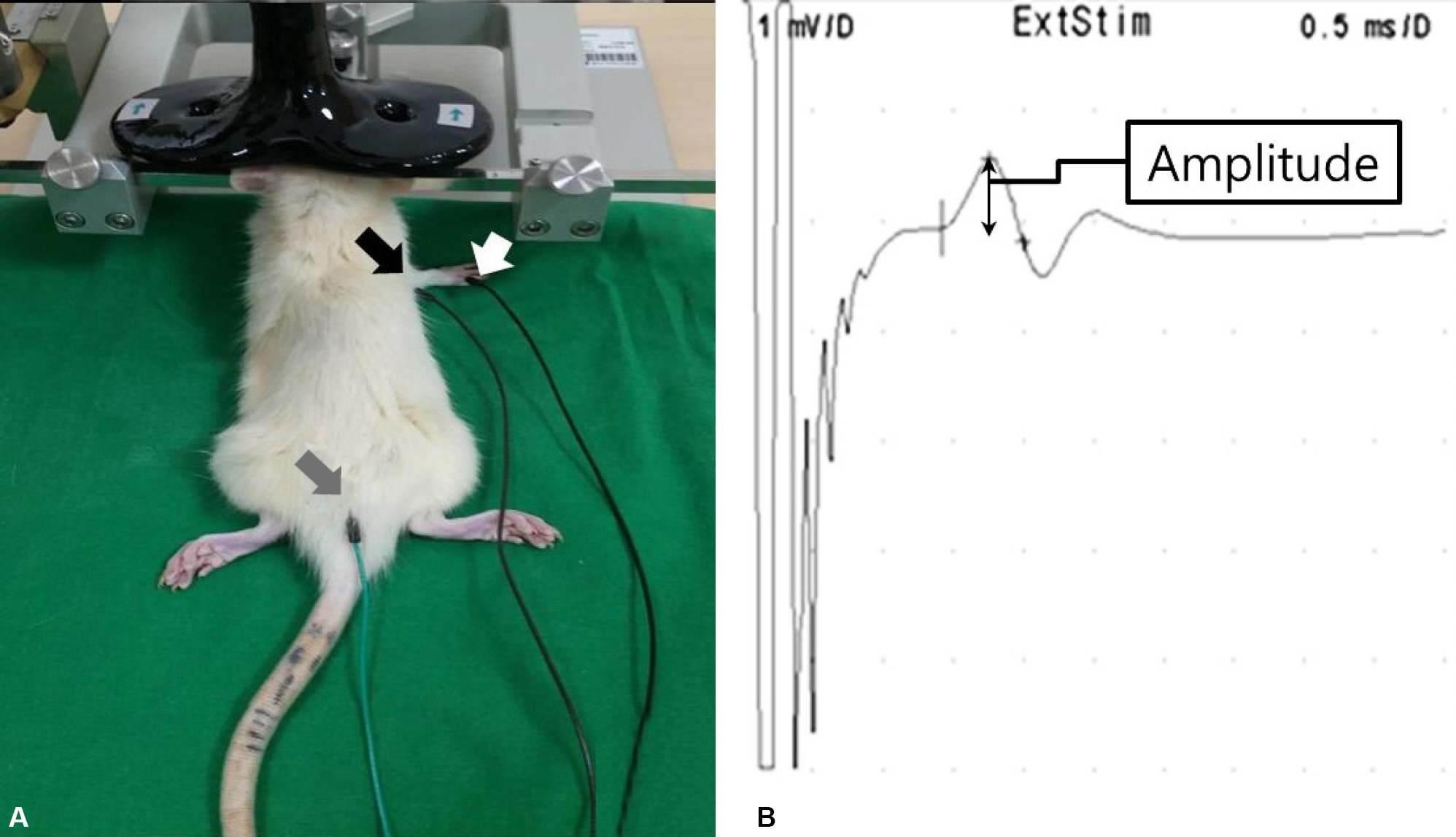
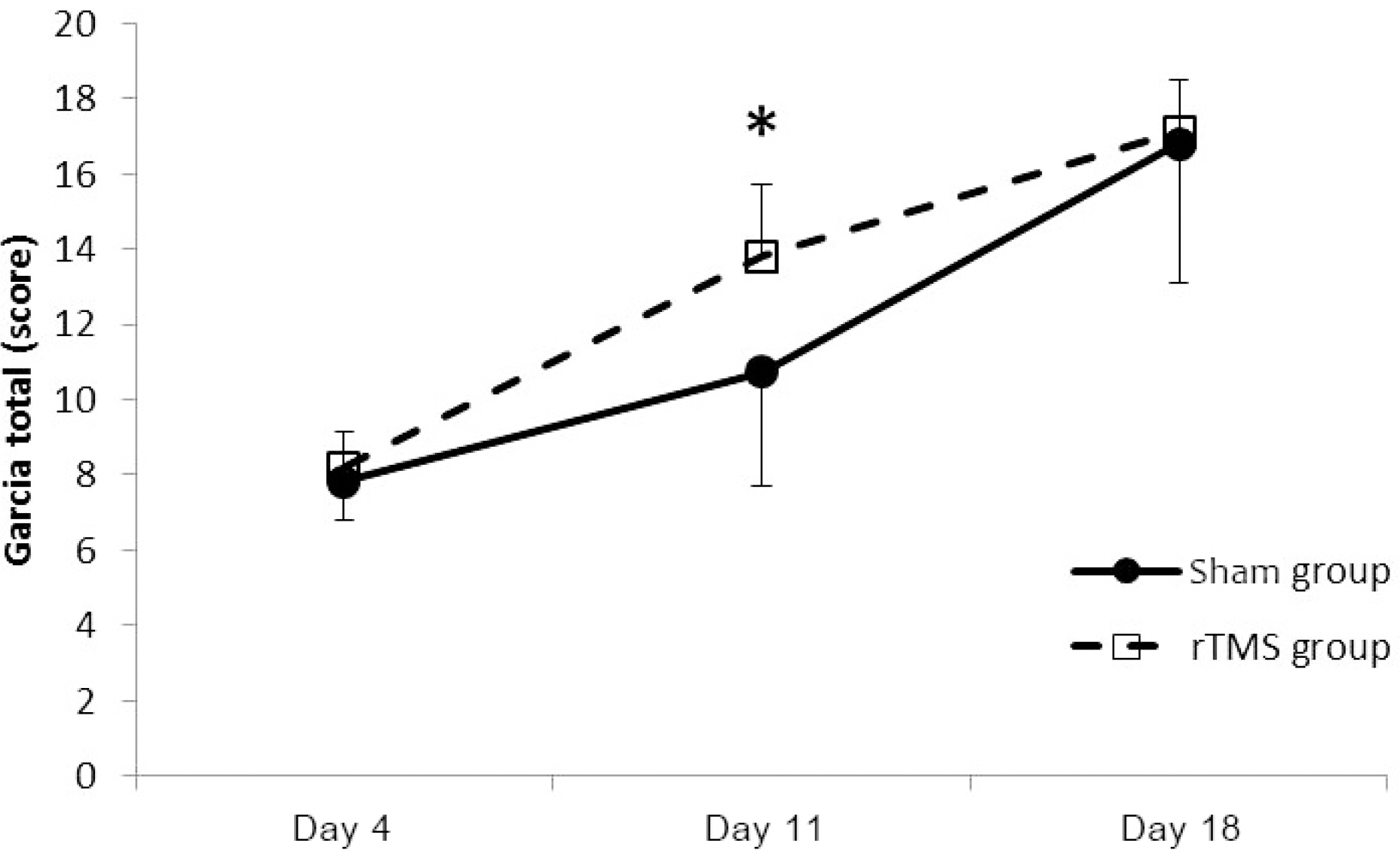
Seventy Sprague-Daley rats were induced permanent middle cerebral artery occlusion (MCAO) stroke model and successful stroke rats (n=56) assigned to the rTMS (n=28) and sham (n=28) group. The 10 Hz, high frequency rTMS gave on ipsilesional forepaw motor cortex during 2 weeks in rTMS group. The somatosensory evoked potential (SSEP) and motor evoked potential (MEP) were used to evaluate the electrophysiological changes. Behavioral function of the stroke rat was evaluated by the Rota rod and Garcia test.
RESULTS
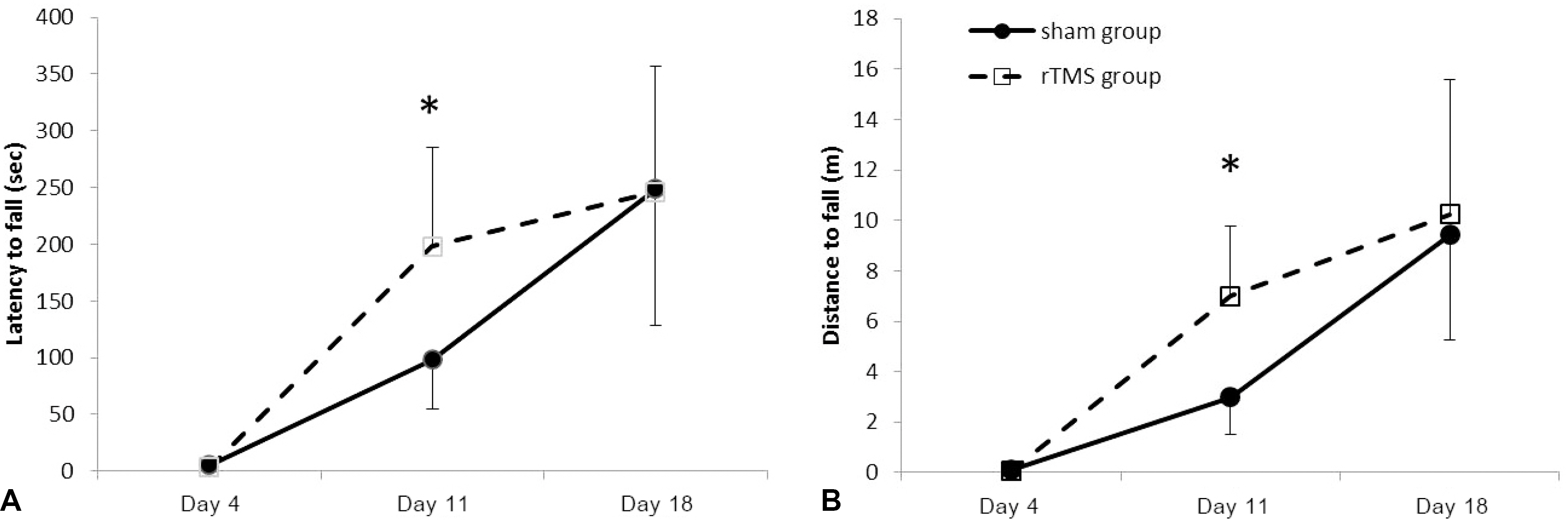
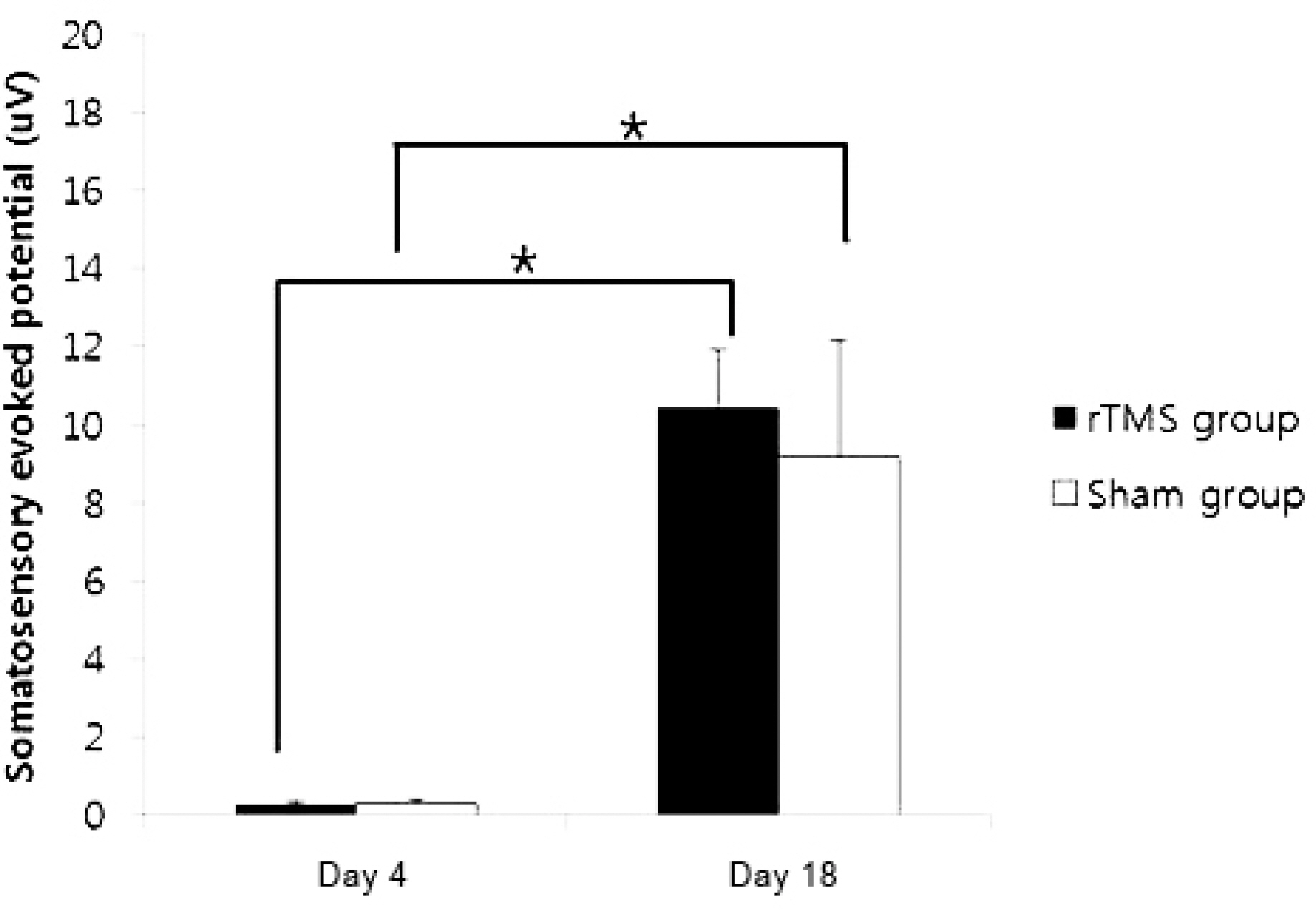
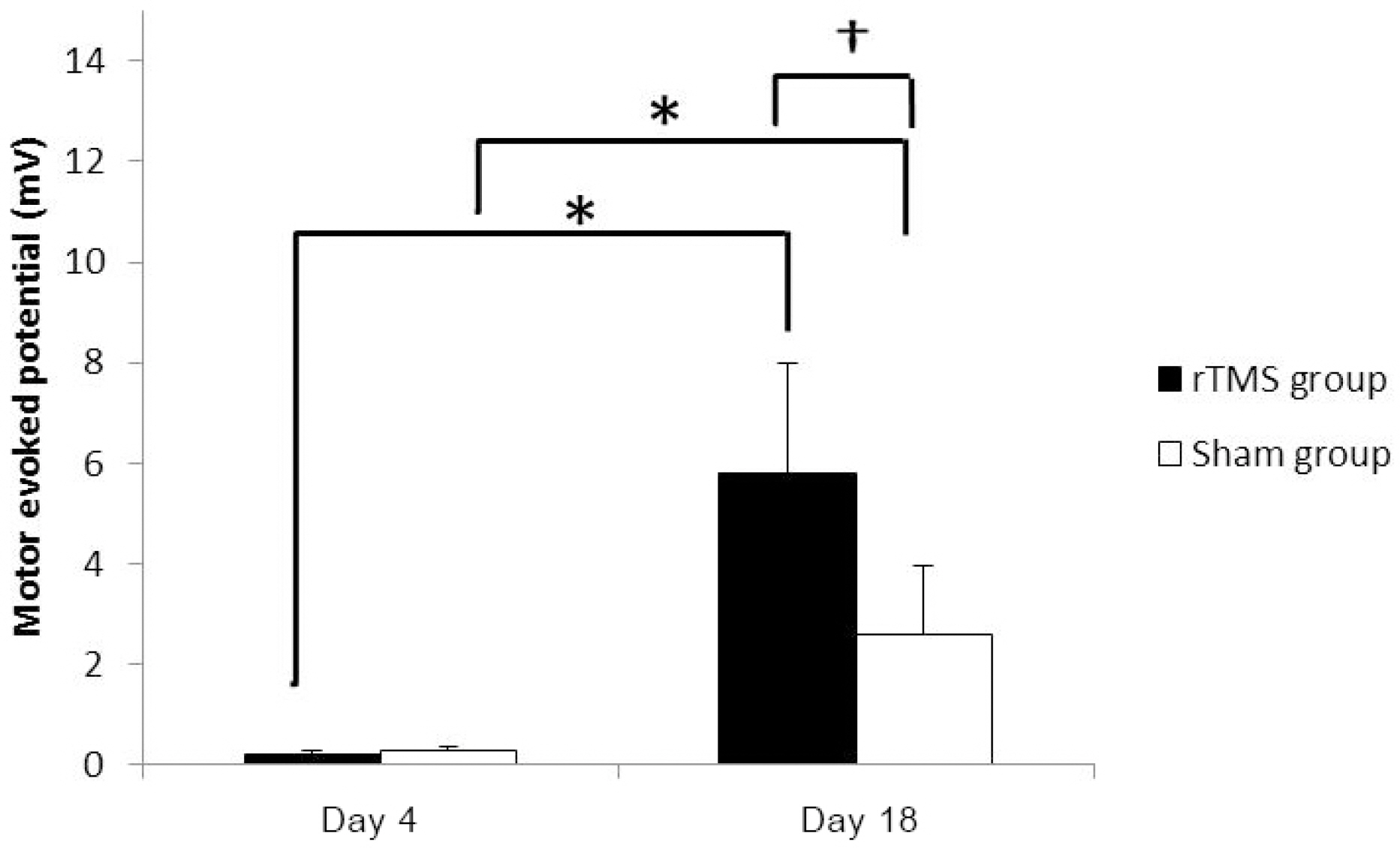
Forty rats (N(rTMS)=20; N(sham)=20) completed all experimental course. The rTMS group showed better performance than sham group in Rota rod test and Garcia test at day 11 (p<0.05) but not day 18 (p>0.05). The amplitude of MEP and SSEP in rTMS group was larger than sham group at day 18 (p<0.05).
CONCLUSIONS
These data confirm that the high frequency rTMS on ipsilesional cerebral motor cortex can help the early recovery of motor performance in permanent middle cerebral artery stroke model and it may simultaneously associate with changes in neurophysiological activity in brain.
MeSH Terms
Figure
Reference
-
1.Walsh V., Desmond JE., Pascual-Leone A. Manipulating brains. Behav Neurol. 2006. 17:131–134.
Article2.Alonso-Alonso M., Fregni F., Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis. 2007. 24:157–166.
Article3.Harvey RL., Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. TopStroke Rehabil. 2007. 14:54–67.
Article4.Minhas AS., Woo EJ., Lee SY. Magnetic flux density measurement with balanced steady state free precession pulse sequence for MREIT: a simulation study. Conf Proc IEEE Eng Med Biol Soc. 2009. 2009:2276–2278.
Article5.Barker AT., Jalinous R., Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985. 1:1106–1107.
Article6.Kobayashi M., Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003. 2:145–156.
Article7.Sokhadze EM., El-Baz AS., Sears LL., Opris I., Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci. 2014. 8:134.
Article8.Maeda F., Keenan JP., Tormos JM., Topka H., Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000. 133:425–430.
Article9.Martin PI., Naeser MA., Theoret H, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004. 25:181–191.
Article10.Kim YH., You SH., Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006. 37:1471–1476.
Article11.Yue L., Xiao-lin H., Tao S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res. 2009. 1260:94–99.
Article12.Muller MB., Toschi N., Kresse AE., Post A., Keck ME. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology. 2000. 23:205–215.13.Kole MH., Fuchs E., Ziemann U., Paulus W., Ebert U. Changes in 5-HT1A and NMDA binding sites by a single rapid transcranial magnetic stimulation procedure in rats. Brain Res. 1999. 826:309–312.
Article14.Antonsson B. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 2001. 306:347–361.
Article15.Fujiki M., Kobayashi H., Abe T., Kamida T. Repetitive transcranial magnetic stimulation for protection against delayed neuronal death induced by transient ischemia. J Neurosurg. 2003. 99:1063–1069.
Article16.Longa EZ., Weinstein PR., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.
Article17.Lee SU., Kim DY., Park SH., Choi DH., Park HW., Han TR. Mild to moderate early exercise promotes recovery from cerebral ischemia in rats. Can J Neurol Sci. 2009. 36:443–449.
Article18.Chen J., Li Y., Wang L., Zhang Z., Lu D., Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001. 32:1005–1011.
Article19.Hamm RJ., Pike BR., O'Dell DM., Lyeth BG., Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994. 11:187–196.
Article20.Garcia JH., Wagner S., Liu KF., Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995. 26:627–634.21.Bazley FA., All AH., Thakor NV., Maybhate A. Plasticity associated changes in cortical somatosensory evoked potentials following spinal cord injury in rats. Conf Proc IEEE Eng Med Biol Soc. 2011. 2005–2008.
Article22.Agrawal G., Thakor NV., All AH. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J Clin Neurosci. 2009. 16:1052–1055.
Article23.Agrawal G., Kerr C., Thakor NV., All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine. 2010. 35:1122–1127.
Article24.All AH., Agrawal G., Walczak P., Maybhate A., Bulte JW., Kerr DA. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurol Sci. 2010. 31:595–601.
Article25.Rotenberg A., Muller PA., Vahabzadeh-Hagh AM., Navarro X., López-Vales R., Pascual-Leone A, et al. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin Neurophysiol. 2010. 121:104–108.
Article26.Yoon KJ., Lee YT., Han TR. Mechanism of functional recovery after repetitive transcranial magnetic stimulation (rTMS) in the subacute cerebral ischemic rat model: neural plasticity or anti-apoptosis? Exp Brain Res. 2011. 214:549–556.
Article27.Kim SJ., Kim BK., Ko YJ., Bang MS., Kim MH., Han TR. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci. 2010. 25:1499–1505.
Article28.Nitsche MA., Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001. 57:1899–1901.
Article