J Korean Soc Radiol.
2014 Sep;71(3):139-145. 10.3348/jksr.2014.71.3.139.
Synchronous Encapsulated Papillary Carcinoma and Invasive Ductal Carcinoma Arising from Intraductal Papilloma in the Same Breast: A Case Report
- Affiliations
-
- 1Department of Radiology, Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea. taloo@hanmail.net
- 2Department of Pathology, Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Nuclear Medicine, Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 4Department of Surgery, Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 1736480
- DOI: http://doi.org/10.3348/jksr.2014.71.3.139
Abstract
- Encapsulated papillary carcinoma of the breast is rare, accounting for just 0.5% to 2% of all breast cancers. A histological upgrade from papillary lesion can also possibly occur, however, an upgrade to invasive ductal carcinoma has uncommonly been reported. Furthermore, to the best of our knowledge, there is no reported case of encapsulated papillary carcinoma and invasive ductal carcinoma arising from intraductal papilloma in the same breast. We report an extremely rare case of synchronous encapsulated papillary carcinoma and invasive ductal carcinoma arising from intraductal papilloma on the same breast with radiologic-pathologic correlation.
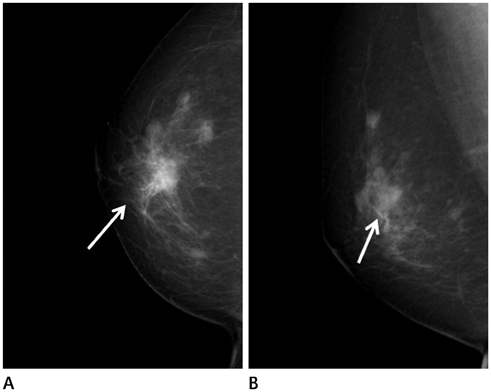
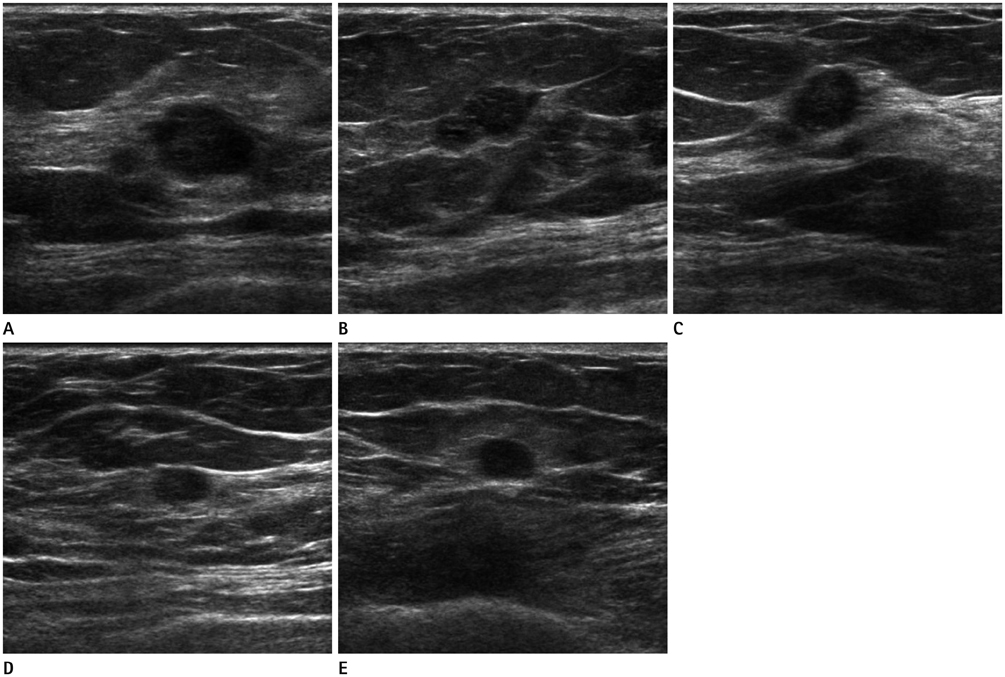
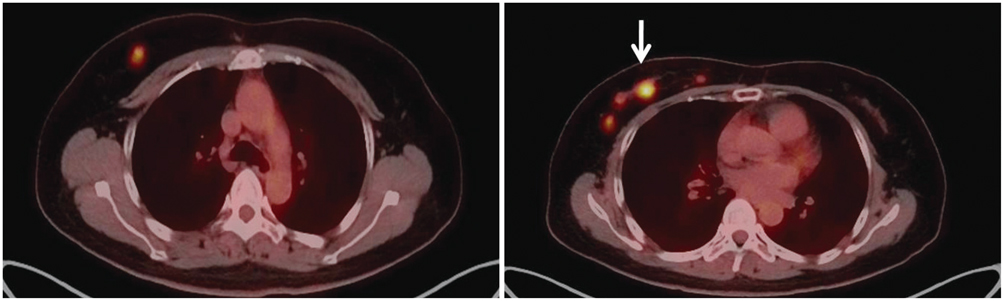
Figure
Reference
-
1. Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006; 238:801–808.2. Liberman L, Tornos C, Huzjan R, Bartella L, Morris EA, Dershaw DD. Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? AJR Am J Roentgenol. 2006; 186:1328–1334.3. Chang JM, Moon WK, Cho N, Han W, Noh DY, Park IA, et al. Risk of carcinoma after subsequent excision of benign papilloma initially diagnosed with an ultrasound (US)-guided 14-gauge core needle biopsy: a prospective observational study. Eur Radiol. 2010; 20:1093–1100.4. Esposito NN, Dabbs DJ, Bhargava R. Are encapsulated papillary carcinomas of the breast in situ or invasive? A basement membrane study of 27 cases. Am J Clin Pathol. 2009; 131:228–224.5. Liberman L. Clinical management issues in percutaneous core breast biopsy. Radiol Clin North Am. 2000; 38:791–807.6. MacGrogan G, Tavassoli FA. Central atypical papillomas of the breast: a clinicopathological study of 119 cases. Virchows Arch. 2003; 443:609–617.7. Rosen PP. Rosen's breast pathology. Philadelphia, PA: Lippincott-Raven;1997. p. 335–354.8. Carter D, Orr SL, Merino MJ. Intracystic papillary carcinoma of the breast. After mastectomy, radiotherapy or excisional biopsy alone. Cancer. 1983; 52:14–11.9. Soo MS, Williford ME, Walsh R, Bentley RC, Kornguth PJ. Papillary carcinoma of the breast: imaging findings. AJR Am J Roentgenol. 1995; 164:321–326.10. Bhatia A, Nahar Saikia Uma, Kumar Y. Rare coexistence of invasive papillary carcinoma with infiltrating ductal carcinoma in male breast: report of a case. Int J Surg Pathol. 2008; 16:311–313.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- The Galectin 3 Expression in Benign and Malignant Breast Tumor
- Invasive Ductal Carcinoma Arising within a Mammary Hamartoma: Case Report
- A Case of Central Nervous System Toxicity Assoclated with Cyclosporine
- Invasive Micropapillary Carcinoma in Axillary Ectopic Breast and Synchronous Ductal Carcinoma In Situ in the Contralateral Breast