Hepatic Arterial Phase on Gadoxetic Acid-Enhanced Liver MR Imaging: A Randomized Comparison of 0.5 mL/s and 1 mL/s Injection Rates
- Affiliations
-
- 1Department of Radiology, Chonnam National University Hwasun Hospital, Hwasun 519-763, Korea. yjeong@jnu.ac.kr
- 2Department of Radiology, Chonnam National University Hospital, Gwangju 501-757, Korea.
- KMID: 1734945
- DOI: http://doi.org/10.3348/kjr.2014.15.5.605
Abstract
OBJECTIVE
To compare gadoxetic acid injection rates of 0.5 mL/s and 1 mL/s for hepatic arterial-phase magnetic resonance (MR) imaging.
MATERIALS AND METHODS
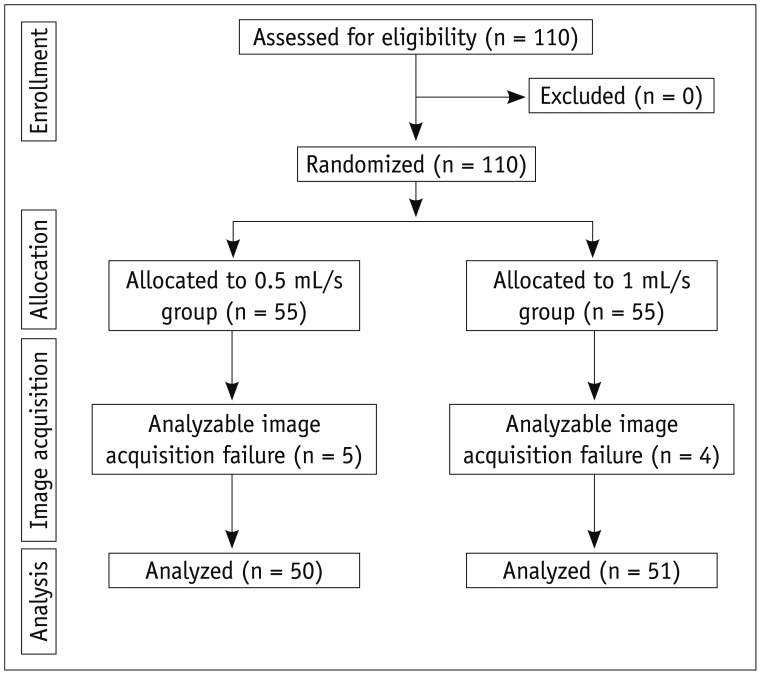
In this prospective study, 101 consecutive patients with suspected focal liver lesions were included and randomly divided into two groups. Each group underwent dynamic liver MR imaging using a 3.0-T scanner after an intravenous injection of gadoxetic acid at rates of either 0.5 mL/s (n = 50) or 1 mL/s (n = 51). Arterial phase images were analyzed after blinding the injection rates. The signal-to-noise ratios (SNRs) of the liver, aorta, portal vein, hepatic vein, spleen, and pancreas were measured. The contrast-to-noise ratios (CNRs) of the hepatocellular carcinomas (HCC) were calculated. Finally, two experienced radiologists were independently asked to identify, if any, HCCs in the liver on the images and score the image quality in terms of the presence of artifacts and the proper enhancement of the liver, aorta, portal vein, hepatic vein, hepatic artery, spleen, pancreas, and kidney.
RESULTS
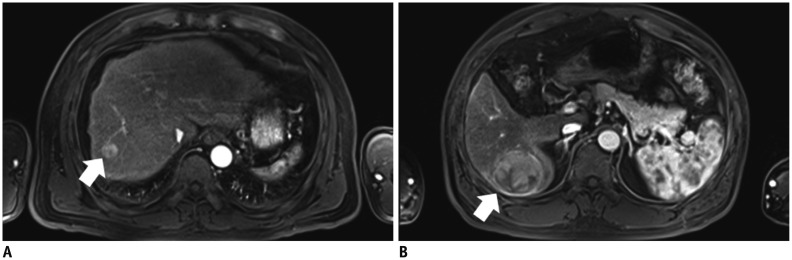
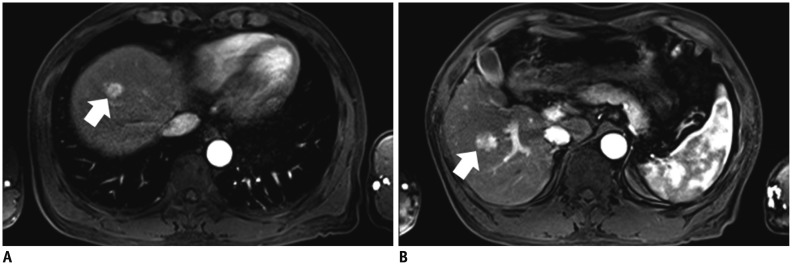
The SNRs were not significantly different between the groups (p = 0.233-0.965). The CNRs of the HCCs were not significantly different (p = 0.597). The sensitivity for HCC detection and the image quality scores were not significantly different between the two injection rates (p = 0.082-1.000).
CONCLUSION
Image quality and sensitivity for hepatic HCCs of arterial-phase gadoxetic acid-enhanced MR were not significantly improved by reducing the contrast injection rate to 0.5 mL/s compared with 1 mL/s.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Artifacts
Carcinoma, Hepatocellular/*radiography
Contrast Media/*administration & dosage/*diagnostic use
Dose-Response Relationship, Drug
Female
Gadolinium DTPA/*administration & dosage/*diagnostic use
Hepatic Artery
Humans
Liver Neoplasms/*radiography
*Magnetic Resonance Imaging
Male
Middle Aged
Prospective Studies
Sensitivity and Specificity
Signal-To-Noise Ratio
Contrast Media
Gadolinium DTPA
Figure
Cited by 2 articles
-
Triple Arterial Phase MR Imaging with Gadoxetic Acid Using a Combination of Contrast Enhanced Time Robust Angiography, Keyhole, and Viewsharing Techniques and Two-Dimensional Parallel Imaging in Comparison with Conventional Single Arterial Phase
Jeong Hee Yoon, Jeong Min Lee, Mi Hye Yu, Eun Ju Kim, Joon Koo Han
Korean J Radiol. 2016;17(4):522-532. doi: 10.3348/kjr.2016.17.4.522.Quantitative Measurement of Hepatic Fibrosis with Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Patients with Chronic Hepatitis B Infection: A Comparative Study on Aspartate Aminotransferase to Platelet Ratio Index and Fibrosis-4 Index
Guy Mok Lee, Youe Ree Kim, Jong Hyun Ryu, Tae-Hoon Kim, Eun Young Cho, Young Hwan Lee, Kwon-Ha Yoon
Korean J Radiol. 2017;18(3):444-451. doi: 10.3348/kjr.2017.18.3.444.
Reference
-
1. Jang HJ, Yu H, Kim TK. Imaging of focal liver lesions. Semin Roentgenol. 2009; 44:266–282. PMID: 19715792.
Article2. Ba-Ssalamah A, Uffmann M, Saini S, Bastati N, Herold C, Schima W. Clinical value of MRI liver-specific contrast agents: a tailored examination for a confident non-invasive diagnosis of focal liver lesions. Eur Radiol. 2009; 19:342–357. PMID: 18810454.
Article3. Bartolozzi C, Cioni D, Donati F, Lencioni R. Focal liver lesions: MR imaging-pathologic correlation. Eur Radiol. 2001; 11:1374–1388. PMID: 11519546.
Article4. Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995; 195:785–792. PMID: 7754011.
Article5. Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996; 199:177–183. PMID: 8633143.
Article6. Vogl TJ, Kümmel S, Hammerstingl R, Schellenbeck M, Schumacher G, Balzer T, et al. Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology. 1996; 200:59–67. PMID: 8657946.
Article7. Reimer P, Rummeny EJ, Daldrup HE, Hesse T, Balzer T, Tombach B, et al. Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol. 1997; 7:275–280. PMID: 9038130.
Article8. Huppertz A, Balzer T, Blakeborough A, Breuer J, Giovagnoni A, Heinz-Peer G, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology. 2004; 230:266–275. PMID: 14695400.
Article9. Bluemke DA, Sahani D, Amendola M, Balzer T, Breuer J, Brown JJ, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology. 2005; 237:89–98. PMID: 16126918.
Article10. Huppertz A, Haraida S, Kraus A, Zech CJ, Scheidler J, Breuer J, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT--initial observations. Radiology. 2005; 234:468–478. PMID: 15591431.
Article11. Halavaara J, Breuer J, Ayuso C, Balzer T, Bellin MF, Blomqvist L, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT--a multicenter trial. J Comput Assist Tomogr. 2006; 30:345–354. PMID: 16778605.12. Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008; 18:457–467. PMID: 18058107.
Article13. Raman SS, Leary C, Bluemke DA, Amendola M, Sahani D, McTavish JD, et al. Improved characterization of focal liver lesions with liver-specific gadoxetic acid disodium-enhanced magnetic resonance imaging: a multicenter phase 3 clinical trial. J Comput Assist Tomogr. 2010; 34:163–172. PMID: 20351497.14. Weinmann HJ, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984; 142:619–624. PMID: 6607655.
Article15. Brasch RC, Weinmann HJ, Wesbey GE. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol. 1984; 142:625–630. PMID: 6607656.
Article16. Hendrick RE, Haacke EM. Basic physics of MR contrast agents and maximization of image contrast. J Magn Reson Imaging. 1993; 3:137–148. PMID: 8428081.
Article17. Brugières P, Gaston A, Degryse HR, Parizel PM, de Schepper AM, Berry I, et al. Randomised double blind trial of the safety and efficacy of two gadolinium complexes (Gd-DTPA and Gd-DOTA). Neuroradiology. 1994; 36:27–30. PMID: 8107991.
Article18. Chung SH, Kim MJ, Choi JY, Hong HS. Comparison of two different injection rates of gadoxetic acid for arterial phase MRI of the liver. J Magn Reson Imaging. 2010; 31:365–372. PMID: 20099350.
Article19. Schmid-Tannwald C, Herrmann K, Oto A, Panteleon A, Reiser M, Zech C. Optimization of the dynamic, Gd-EOB-DTPA-enhanced MRI of the liver: the effect of the injection rate. Acta Radiol. 2012; 53:961–965. PMID: 23024179.
Article20. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009; 44:305–310. PMID: 19462484.
Article21. Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005; 40:715–724. PMID: 16230904.
Article22. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.
Article23. Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001; 34:570–575. PMID: 11394657.24. Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol. 2009; 15:391–423. PMID: 19783891.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic Angiomyolipoma Presenting as a Hyperintense Lesion During the Hepatobiliary Phase of Gadoxetic Acid Enhanced-MRI: a Case Report
- Triple Arterial Phase MR Imaging with Gadoxetic Acid Using a Combination of Contrast Enhanced Time Robust Angiography, Keyhole, and Viewsharing Techniques and Two-Dimensional Parallel Imaging in Comparison with Conventional Single Arterial Phase
- Evaluation and Prediction of Post-Hepatectomy Liver Failure Using Imaging Techniques: Value of Gadoxetic Acid-Enhanced Magnetic Resonance Imaging
- Current Limitations and Potential Breakthroughs for the Early Diagnosis of Hepatocellular Carcinoma
- Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers