J Korean Med Sci.
2014 Mar;29(3):386-391. 10.3346/jkms.2014.29.3.386.
18F-FDG Positron-Emission Tomography/Computed Tomography Findings of Radiographic Lesions Suggesting Old Healed Tuberculosis
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- 2Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine and Lung Institute of Medical Research Center, Seoul National University College of Medicine, Seoul, Korea. yimjj@snu.ac.kr
- KMID: 1734926
- DOI: http://doi.org/10.3346/jkms.2014.29.3.386
Abstract
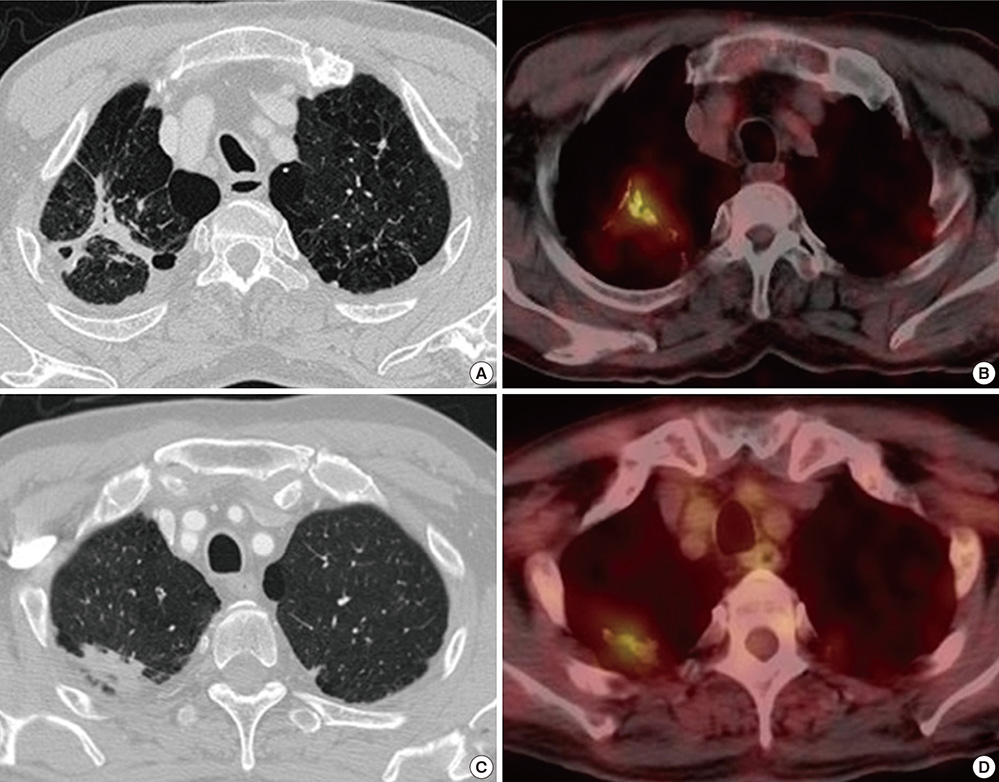
- The presence of radiographic lesions suggesting old healed tuberculosis (TB) is one of the strongest risk factors for the subsequent development of active TB. We elucidated the metabolic activity of radiographic lesions suggesting old healed TB using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). This cross-sectional study included 63 participants with radiographic lesions suggesting old healed TB and with available 18F-FDG PET/CT scans. The maximum standardized uptake value (SUVmax) measured in the lesions, the clinical characteristics, results of the tuberculin skin test (TST) and interferon-gamma release assay (IGRA) were analyzed. The SUVmax in old healed TB was 1.5 or higher among nine (14.3%) participants. Age (adjusted odds ratio [aOR], 1.23; 95% CI, 1.03-1.46), history of previous TB (aOR, 60.43; 95% CI, 1.71-2131.65), and extent of the lesions (aOR, 1.34; 95% CI, 1.02-1.75) were associated with higher SUVmax. The positive rates for the TST and IGRA were not different between groups with and without increased FDG uptake. Increased FDG uptake on 18F-FDG PET/CT was observed in a subset of patients with radiographic lesions suggesting old healed TB. Given that the factors associated with increased FDG uptake are known risk factors for TB development, the possibility exists that participants with old healed TB lesions with higher SUV on 18F-FDG PET/CT scans might be at higher risk for active TB.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Cross-Sectional Studies
Diagnosis, Differential
Female
Fluorodeoxyglucose F18/chemistry/*diagnostic use
Follow-Up Studies
Humans
Interferon-gamma Release Tests
Male
Middle Aged
Odds Ratio
Positron-Emission Tomography
Radiopharmaceuticals/chemistry/*diagnostic use
Risk Factors
Tomography, X-Ray Computed
Tuberculin Test
Tuberculosis/*diagnosis/radiography
Fluorodeoxyglucose F18
Radiopharmaceuticals
Figure
Reference
-
1. Kumar V, Abbas AK, Fausto N, Mitchell R. Robbins basic pathology. 8th ed. Philadelphia: Saunders Elsevier;2007. p. 516–522.2. Horsburgh CR Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004; 350:2060–2067.3. Knight SB, Delbeke D, Stewart JR, Sandler MP. Evaluation of pulmonary lesions with FDG-PET: comparison of findings in patients with and without a history of prior malignancy. Chest. 1996; 109:982–988.4. Dewan NA, Gupta NC, Redepenning LS, Phalen JJ, Frick MP. Diagnostic efficacy of PET-FDG imaging in solitary pulmonary nodules: potential role in evaluation and management. Chest. 1993; 104:997–1002.5. Basu S, Saboury B, Werner T, Alavi A. Clinical utility of FDG-PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol. 2011; 13:1051–1060.6. Ichiya Y, Kuwabara Y, Sasaki M, Yoshida T, Akashi Y, Murayama S, Nakamura K, Fukumura T, Masuda K. FDG-PET in infectious lesions: the detection and assessment of lesion activity. Ann Nucl Med. 1996; 10:185–191.7. Shim SS, Lee KS, Kim BT, Choi JY, Chung MJ, Lee EJ. Focal parenchymal lung lesions showing a potential of false-positive and false-negative interpretations on integrated PET/CT. AJR Am J Roentgenol. 2006; 186:639–648.8. Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992; 33:1972–1980.9. Jones HA, Clark RJ, Rhodes CG, Schofield JB, Krausz T, Haslett C. In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med. 1994; 149:1635–1639.10. Brudin LH, Valind SO, Rhodes CG, Pantin CF, Sweatman M, Jones T, Hughes JM. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994; 21:297–305.11. Yang CM, Hsu CH, Lee CM, Wang FC. Intense uptake of [F-18]-fluoro-2 deoxy-D-glucose in active pulmonary tuberculosis. Ann Nucl Med. 2003; 17:407–410.12. Bakheet SM, Powe J, Ezzat A, Rostom A. F-18-FDG uptake in tuberculosis. Clin Nucl Med. 1998; 23:739–742.13. Yen RF, Chen ML, Liu FY, Ko SC, Chang YL, Chieng PU, Su CT. False-positive 2-[F-18]-fluoro-2-deoxy-D-glucose positron emission tomography studies for evaluation of focal pulmonary abnormalities. J Formos Med Assoc. 1998; 97:642–645.14. Knopp MV, Bischoff HG. Evaluation of pulmonary lesions with positron emission tomography. Radiologe. 1994; 34:588–591.15. Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, Chung JK. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology. 2000; 216:117–121.16. Kim IJ, Lee JS, Kim SJ, Kim YK, Jeong YJ, Jun S, Nam HY, Kim JS. Double-phase 18F-FDG PET-CT for determination of pulmonary tuberculoma activity. Eur J Nucl Med Mol Imaging. 2008; 35:808–814.17. Hahm CR, Park HY, Jeon K, Um SW, Suh GY, Chung MP, Kim H, Kwon OJ, Koh WJ. Solitary pulmonary nodules caused by Mycobacterium tuberculosis and Mycobacterium avium complex. Lung. 2010; 188:25–31.18. Park IN, Ryu JS, Shim TS. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008; 33:1–3.19. Martinez V, Castilla-Lievre MA, Guillet-Caruba C, Grenier G, Fior R, Desarnaud S, Doucet-Populaire F, Boué F. (18)F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis. 2012; 16:1180–1185.20. Martinez VN, Komatsu NK, De Figueredo SM, Waldman EA. Equity in health: tuberculosis in the Bolivian immigrant community of São Paulo, Brazil. Trop Med Int Health. 2012; 17:1417–1424.21. Linh NN, Marks GB, Crawford AB. Radiographic predictors of subsequent reactivation of tuberculosis. Int J Tuberc Lung Dis. 2007; 11:1136–1142.22. Targeted tuberculin testing and treatment of latent tuberculosis infection: this official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999: this is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC): this statement was endorsed by the Council of the Infectious Diseases Society of America (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161:S221–S247.23. Yu CH, Wang T, Sun YE, Yao SL, Tian JH, Yin DY. Fluorine-18 fluorodeoxyglucose uptake in patients with benign pulmonary nodules. Zhonghua Wai Ke Za Zhi. 2006; 44:90–92.24. Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012; 12:581–591.25. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009; 15:211–214.26. Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011; 43:482–486.27. Horsburgh CR Jr, O'Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, Narita M, Johnson LS, von Reyn CF. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. 2010; 182:420–425.28. Stead WW, Lofgren JP. Does the risk of tuberculosis increase in old age? J Infect Dis. 1983; 147:951–955.29. Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999; 3:S231–S279.30. Cao JP, Zhang LY, Zhu JQ, Chin DP. Two-year follow-up of directly-observed intermittent regimens for smear-positive pulmonary tuberculosis in China. Int J Tuberc Lung Dis. 1998; 2:360–364.31. Thomas A, Gopi PG, Santha T, Chandrasekaran V, Subramani R, Selvakumar N, Eusuff SI, Sadacharam K, Narayanan PR. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis. 2005; 9:556–561.32. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial: International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982; 60:555–564.33. Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008; 177:1164–1170.34. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012; 142:63–75.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-Malignant 18F-FDG Uptake in the Thorax by Positron Emission Tomography Computed Tomography Fusion Imaging
- 18F-FDG PET/CT Findings in a Breast Cancer Patient with Concomitant Tuberculous Axillary Lymphadenitis
- A Comparison Study of Esophageal Findings on 18F-FDG PET/CT and Esophagogastroduodenoscopy
- Perirenal 18F-FDG Uptake in a Patient with a Pheochromocytoma
- Detecting Metastatic Bladder Cancer Using 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography