J Korean Med Sci.
2004 Oct;19(5):750-752. 10.3346/jkms.2004.19.5.750.
A Case Report of a Poor Metabolizer of CYP2D6 Presented with Unusual Responses to Nortriptyline Medication
- Affiliations
-
- 1Department of Laboratory Medicine, Sungkyunkwan University School of Medicine Samsung Medical Center, Seoul, Korea. jwonk@smc.samsung.co.kr
- 2Department of Psychiatry, Sungkyunkwan University School of Medicine Samsung Medical Center, Seoul, Korea.
- KMID: 1733521
- DOI: http://doi.org/10.3346/jkms.2004.19.5.750
Abstract
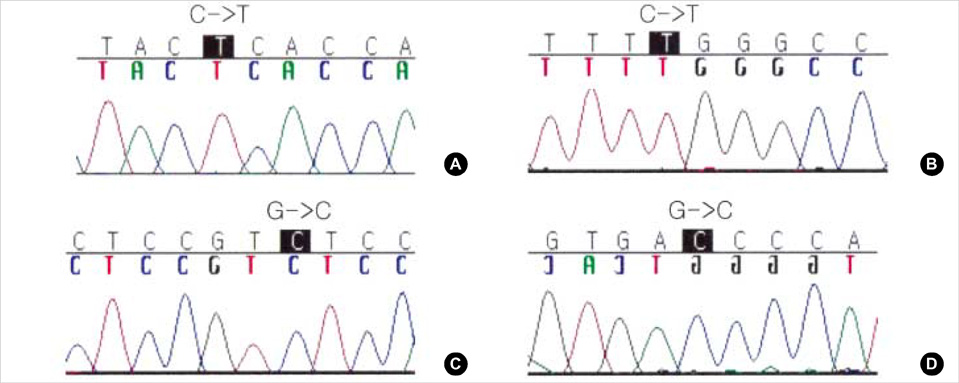
- We present a case with decreased metabolic activity of CYP2D6, a cytochrome P450 enzyme catalyzing the metabolism of nortriptyline (NT). Conventional dosage regimen led to toxic plasma concentration of NT and adverse effects such as dry mouth, constipation, and dizziness in this case with genotype CYP2D6*5/*10B. This case suggests the clinical usefulness of pharmacogenetic testing in individualized dosage adjustments of NT.
MeSH Terms
Figure
Reference
-
1. Preskorn SH. Pharmacokinetics of antidepressants: why and how they are relevant to treatment. J Clin Psychiatry. 1993. 54:14–34.2. Linder MW, Keck PE Jr. Standards of laboratory practice: antidepressant drug monitoring. Clin Chem. 1998. 44:1073–1084.3. Morita S, Shimoda K, Someya T, Yoshimura Y, Kamijima K, Kato N. Steady state plasma levels of nortriptyline and its hydroxylated metabolites in Japanese patients: Impact of CYP2D6 genotype on the hydroxylation of nortriptyline. J Clin Psychopharmacol. 2000. 20:141–149.4. Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002. 53:111–122.
Article5. Dahl ML, Bertilsson L, Nordin C. Steady-state plasma levels of nortriptyline and its 10-hydroxy metabolite: relationship to the CYP2D6 genotype. Psychopharmacology. 1996. 123:315–319.6. Dalen P, Dahl ML, Ruiz ML, Nordin J, Bertilsson L. 10-hydroxylation of nortriptyline in white persons with 0, 1, 2, 3 and 13 functional CYP2D6 genes. Clin Pharmacol Ther. 1998. 63:444–452.
Article7. Bertilsson L, Mellstrom B, Sjokvist F, Martension B, Asberg M. Slow hydroxylation of nortriptyline and concomitant poor debrisoquine hydroxylation: clinical implications: letter. Lancet. 1981. 1:560–561.8. Kimura S, Umeno M, Skoda RC, Meyer UA, Gonzalez FJ. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989. 45:889–894.9. Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E. Rapid detection of the CYP2D6*3, CYP2D6*4, CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem. 2000. 46:1072–1077.10. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. 2002. 3:229–243.
Article11. Kubota T, Yamaura Y, Ohkawa N, Hara H, Chiba K. Frequencies of CYP2D6 mutant alleles in a normal Japanese population and metabolic activity of dextromethorphan o-demethylation in different CYP2D6 genotypes. Br J Clin Pharmacol. 2000. 50:31–34.12. Roh HK, Dahl ML, Johansson I, Ingelman-Sundberg M, Cha YN, Bertilsson L. Debrisoquine and S-mephenytoin hydroxylation phenotypes and genotypes in a Korean population. Pharmacogenetics. 1996. 6:441–447.
Article13. Shim JC, Gong B, Park JH, Yoon YR, Sin JG, Kim JI, Ahn DS, Kim YK, Cha IJ, Kim YH. Combined therapy of paroxetine and tricyclic antidepressant in depression of schizophrenic patients. J Korean Neuropsychiatr Assoc. 1997. 36:732–741.14. DeVane CL, Nemeroff CB. An evaluation of risperidone drug interactions. J Clin Psychopharmacol. 2001. 21:408–416.
Article15. Franssen EJ, Kunst PW, Bet PM, Strack van Schijndel RJ, van Loenen AC, Wilhelm AJ. Toxicokinetics of nortriptyline and amitriptyline: two case reports. Ther Drug Monit. 2003. 25:248–251.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antipsychotics Induced Etrapyramidal Symptoms in Schizophrenics in Relation to Cytochrome P450 2D6 Genotype
- CYP2D6 and NAT2 Polymorphism in HBV Associated in Hepatocellular Carcinoma Patients in Korea
- Association between Genetic Polymorphisms of CYP2D6 and Outcomes in Breast Cancer Patients with Tamoxifen Treatment
- The effect ofsingle oral dose of nortriptyline on plasma 3-methoxy-4-hydroxyphenethyleneglycol in healthy subjects
- Population Pharmacokinetic− Pharmacodynamic Modeling of Carvedilol to Evaluate the Effect of Cytochrome P450 2D6 Genotype on the Heart Rate Reduction