Korean J Radiol.
2014 Aug;15(4):523-529. 10.3348/kjr.2014.15.4.523.
Altered Activity and Functional Connectivity of Superior Temporal Gyri in Anxiety Disorders: A Functional Magnetic Resonance Imaging Study
- Affiliations
-
- 1Department of Radiology, Tong Ji Hospital of Tong Ji University, Shanghai 200065, China. tongjipjwang@vip.sina.com
- 2Department of Psychiatry, Tong Ji Hospital of Tong Ji University, Shanghai 200065, China.
- 3Bio-X lab, Department of Physics, Zhe Jiang University, Hangzhou 310027, China.
- KMID: 1731057
- DOI: http://doi.org/10.3348/kjr.2014.15.4.523
Abstract
OBJECTIVE
The prior functional MRI studies have demonstrated significantly abnormal activity in the bilateral superior temporal gyrus (STG) of anxiety patients. The purpose of the current investigation was to determine whether the abnormal activity in these regions was related to a loss of functional connectivity between these regions.
MATERIALS AND METHODS
Ten healthy controls and 10 anxiety patients underwent noninvasive fMRI while actively listening to emotionally neutral words alternated by silence (Task 1) or threat-related words (Task 2). The participants were instructed to silently make a judgment of each word's valence (i.e., unpleasant, pleasant, or neutral). A coherence analysis was applied to the functional MRI data to examine the functional connectivity between the left and the right STG, which was selected as the primary region of interest on the basis of our prior results.
RESULTS
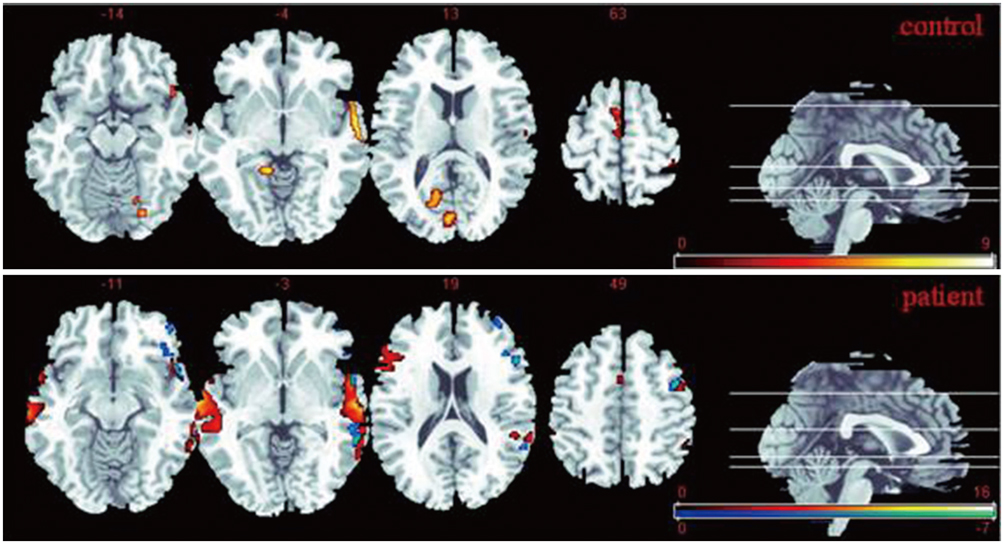
The data demonstrated that the anxiety patients exhibited significantly increased activation in the bilateral STG than the normal controls. The functional connectivity analysis indicated that the patient group showed significantly decreased degree of connectivity between the bilateral STG during processing Task 2 compared to Task 1 (t = 2.588, p = 0.029). In addition, a significantly decreased connectivity was also observed in the patient group compared to the control group during processing Task 2 (t = 2.810, p = 0.012).
CONCLUSION
Anxiety patients may exhibit increased activity of the STG but decreased functional connectivity between the left and right STG, which may reflect the underlying neural abnormality of anxiety disorder, and this will provide new insights into this disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry. 2002; 51:92–100.2. Hermann C, Ofer J, Flor H. Covariation bias for ambiguous social stimuli in generalized social phobia. J Abnorm Psychol. 2004; 113:646–653.3. Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In : Gross JJ, editor. Handbook of Emotion Regulation. New York, NY: Guilford Press;2007. p. 542–559.4. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007; 164:1476–1488.5. Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004; 4:275–284.6. Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res. 2006; 40:709–729.7. Zhao XH, Wang PJ, Li CB, Wang JH, Yang ZY, Hu ZH, et al. Prefrontal and superior temporal lobe hyperactivity as a biological substrate of generalized anxiety disorders. Zhonghua Yi Xue Za Zhi. 2006; 86:955–960.8. Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q, Wu WY, et al. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007; 63:373–378.9. Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003; 19(2 Pt 1):466–470.10. Sporns O. Networks of the Brain. Cambridge, MA: MIT Press;2010. p. 179–201.11. Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008; 131(Pt 4):945–961.12. Delbeuck X, Collette F, Van der Linden M. Is Alzheimer's disease a disconnection syndrome? Evidence from a crossmodal audio-visual illusory experiment. Neuropsychologia. 2007; 45:3315–3323.13. Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993; 13:5–14.14. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995; 34:537–541.15. Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002; 15:247–262.16. Sun FT, Miller LM, D'Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004; 21:647–658.17. Müller K, Lohmann G, Bosch V, von Cramon DY. On multivariate spectral analysis of fMRI time series. Neuroimage. 2001; 14:347–356.18. Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004; 22:63–71.19. Turk CL, Heimberg RG, Luterek JA, Mennin DS, Fresco DM. Emotion dysregulation in generalized anxiety disorder: a comparison with social anxiety disorder. Cognit Ther Res. 2005; 29:89–106.20. Mennin DS, Turk CL, Heimberg RG, Carmin CN. Regulation of emotion in generalized anxiety disorder. In : Reinecke MA, Clark DA, Beck A, editors. Cognitive Therapy over the Lifespan: Theory, Research, and Practice. New York: Cambridge University Press;2003. p. 60–89.21. Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clin Psychol Sci Pract. 2002; 9:85–90.22. Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009; 66:1361–1372.23. Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011; 56:881–889.24. Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997; 19:613–624.25. De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2002; 51:553–562.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Occupational Trauma Exposure on Brain Functional Connectivity in Firefighters With Subclinical Post-Traumatic Stress Symptoms: A Resting-State Functional Magnetic Resonance Imaging Study
- Improved Diagnostic Accuracy of Alzheimer's Disease by Combining Regional Cortical Thickness and Default Mode Network Functional Connectivity: Validated in the Alzheimer's Disease Neuroimaging Initiative Set
- Recent Advances on Resting State Functional Abnormalities of the Default Mode Network in Social Anxiety Disorder
- Functional Magnetic Resonance Imaging of the Brain: Principle and Practical Application
- MRI Study on the Functional and Spatial Consistency of Resting State-Related Independent Components of the Brain Network