Clin Orthop Surg.
2010 Jun;2(2):90-97. 10.4055/cios.2010.2.2.90.
Effect of Hydroxyapatite on Bone Integration in a Rabbit Tibial Defect Model
- Affiliations
-
- 1Department of Orthopedic Surgery, Dong-A University College of Medicine, Busan, Korea. tynitus@dau.ac.kr
- 2Department of Orthopaedic Surgery, Dong-Eui Medical Center, Busan, Korea.
- 3Department of Ophthalmology, Dong-A University College of Medicine, Busan, Korea.
- 4Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Micobiology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 1719335
- DOI: http://doi.org/10.4055/cios.2010.2.2.90
Abstract
- BACKGROUND
The aim of the present study was to prepare hydroxyapatite (HA) and then characterize its effect on bone integration in a rabbit tibial defect model. The bone formation with different designs of HA was compared and the bony integration of several graft materials was investigated qualitatively by radiologic and histologic study.
METHODS
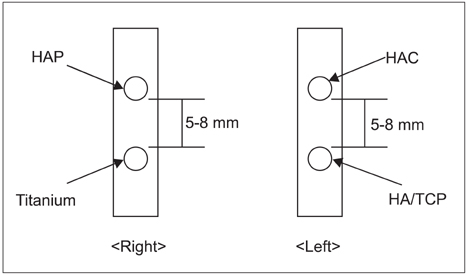
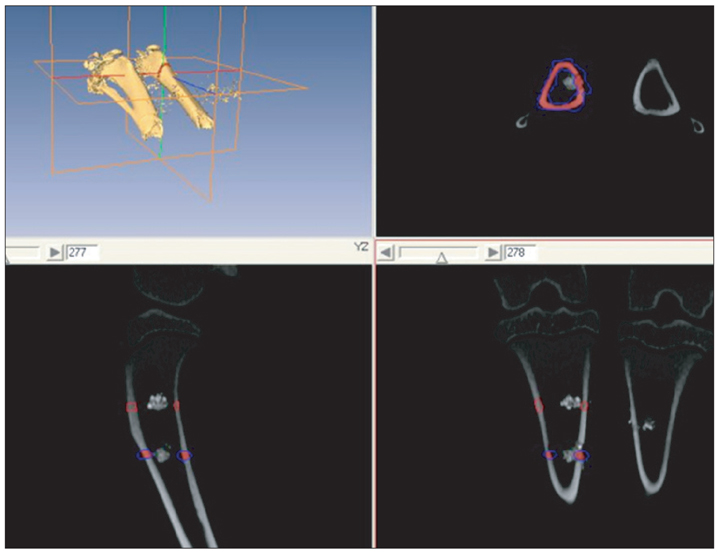
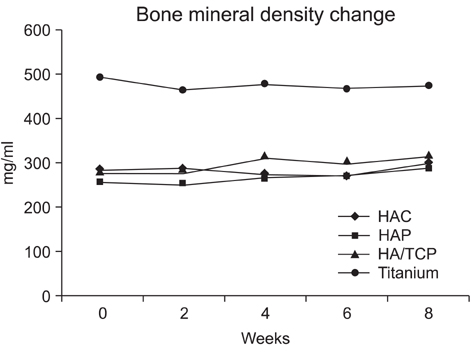
Ten rabbits were included in this study; two holes were drilled bilaterally across the near cortex and the four holes in each rabbit were divided into four treatment groups (HAP, hydroxyapatite powder; HAC, hydroxyapatite cylinder; HA/TCP, hydroxyapatite/tri-calcium phosphate cylinder, and titanium cylinder). The volume of bone ingrowth and the change of bone mineral density were statistically calculated by computed tomography five times for each treatment group at 0, 2, 4, 6, and 8 weeks after grafting. Histologic analysis was performed at 8 weeks after grafting.
RESULTS
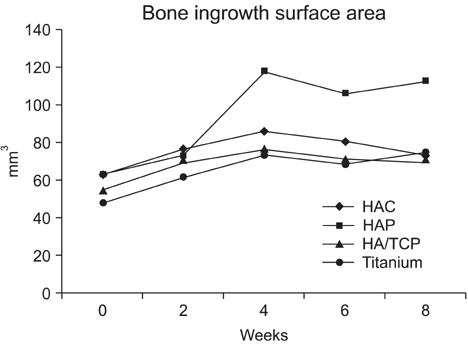
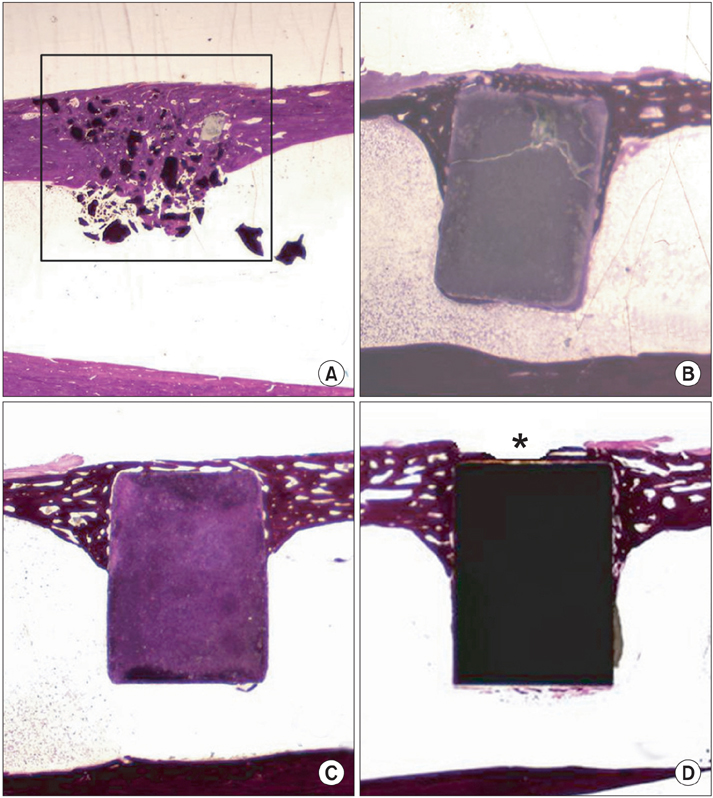
The HAP group showed the most pronounced effect on the bone ingrowth surface area, which seen at 4, 6, and 8 weeks after graft (p < 0.05). On comparing the change of bone mineral density the bone ingrowth surface area among the 4 groups, there were no statistically significant differences among the groups found for any period (p > 0.05). On histological examination, the HAP group revealed well-recovered cortical bone, but the bone was irregularly thickened and haphazardly admixed with powder. The HAC group showed similar histological features to those of the HA/TCP group; the cortical surface of the newly developed bone was smooth and the bone matrix on the surface of the cylinder was regularly arranged.
CONCLUSIONS
We concluded that both the hydroxyapatite powder and cylinder models investigated in our study may be suitable as a bone substitute in the rabbit tibial defect model, but their characteristic properties are quite different. In contrast to hydroxyapatite powder, which showed better results for the bone ingrowth surface, the hydroxyapatite cylinder showed better results for the sustained morphology.
Keyword
MeSH Terms
Figure
Reference
-
1. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996. (329):300–309.
Article2. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft: complications and functional assessment. Clin Orthop Relat Res. 1997. (339):76–81.
Article3. Agrillo U, Mastronardi L, Puzzilli F. Anterior cervical fusion with carbon fiber cage containing coralline hydroxyapatite: preliminary observations in 45 consecutive cases of soft-disc herniation. J Neurosurg. 2002. 96:3 Suppl. 273–276.
Article4. Bucholz RW, Carlton A, Holmes R. Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Clin Orthop Relat Res. 1989. (240):53–62.
Article5. Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine (Phila Pa 1976). 2000. 25(5):563–569.6. Holmes R, Mooney V, Bucholz R, Tencer A. A coralline hydroxyapatite bone graft substitute: preliminary report. Clin Orthop Relat Res. 1984. (188):252–262.7. Keating JF, Hajducka CL, Harper J. Minimal internal fixation and calcium-phosphate cement in the treatment of fractures of the tibial plateau: a pilot study. J Bone Joint Surg Br. 2003. 85(1):68–73.8. Sartoris DJ, Gershuni DH, Akeson WH, Holmes RE, Resnick D. Coralline hydroxyapatite bone graft substitutes: preliminary report of radiographic evaluation. Radiology. 1986. 159(1):133–137.
Article9. Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement: a prospective, randomised study using Norian SRS. J Bone Joint Surg Br. 2000. 82(6):856–863.10. Thalgott JS, Fritts K, Giuffre JM, Timlin M. Anterior interbody fusion of the cervical spine with coralline hydroxyapatite. Spine (Phila Pa 1976). 1999. 24(13):1295–1299.
Article11. Uchida A, Nade SM, McCartney ER, Ching W. The use of ceramics for bone replacement: a comparative study of three different porous ceramics. J Bone Joint Surg Br. 1984. 66(2):269–275.
Article12. Uchida A, Araki N, Shinto Y, Yoshikawa H, Kurisaki E, Ono K. The use of calcium hydroxyapatite ceramic in bone tumour surgery. J Bone Joint Surg Br. 1990. 72(2):298–302.
Article13. Yamamoto T, Onga T, Marui T, Mizuno K. Use of hydroxyapatite to fill cavities after excision of benign bone tumours: clinical results. J Bone Joint Surg Br. 2000. 82(8):1117–1120.14. Cooke FW. Ceramics in orthopedic surgery. Clin Orthop Relat Res. 1992. (276):135–146.
Article15. Boyne PJ. Implant dentistry forefront '85: design and methods. J Oral Implantol. 1986. 12(3):333–337.16. Finn RA, Bell WH, Brammer JA. Interpositional "grafting" with autogenous bone and coralline hydroxyapatite. J Maxillofac Surg. 1980. 8(3):217–227.
Article17. Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects: a histometric study. J Bone Joint Surg Am. 1986. 68(6):904–911.
Article18. Piecuch JF, Topazian RG, Skoly S, Wolfe S. Experimental ridge augmentation with porous hydroxyapatite implants. J Dent Res. 1983. 62(2):148–154.
Article19. Sartoris DJ, Holmes RE, Tencer AF, Mooney V, Resnick D. Coralline hydroxyapatite bone graft substitutes in a canine metaphyseal defect model: radiographic-biomechanical correlation. Skeletal Radiol. 1986. 15(8):635–641.
Article20. Martin RB, Chapman MW, Holmes RE, et al. Effects of bone ingrowth on the strength and non-invasive assessment of a coralline hydroxyapatite material. Biomaterials. 1989. 10(7):481–488.
Article21. Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 1991. 73(5):692–703.
Article22. Katthagen BD. Bone regeneration with bone substitutes: an animal study. 1986. Berlin: Springer-Verlag;47–50.23. Schweiberer L. Experimental studies on bone transplantation with unchanged and denaturated bone substance: a contribution on causal osteogenesis. Hefte Unfallheilkd. 1970. 103(1):1–70.24. David V, Laroche N, Boudignon B, et al. Noninvasive in vivo monitoring of bone architecture alterations in hindlimb-unloaded female rats using novel three-dimensional microcomputed tomography. J Bone Miner Res. 2003. 18(9):1622–1631.
Article25. Waarsing JH, Day JS, van der, et al. Detecting and tracking local changes in the tibiae of individual rats: a novel method to analyse longitudinal in vivo micro-CT data. Bone. 2004. 34(1):163–169.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of a Hydroxyapatite and 4-hexylresorcinol Combination Graft on Bone Regeneration in the Rabbit Calvarial Defect Model
- The Effect of Silk Fibroin/Nano-hydroxyapatite/Corn Starch Composite Porous Scaffold on Bone Regeneration in the Rabbit Calvarial Defect Model
- A Comparative Study on Regeneration of Bone Defects after the Grafts of Demineralized Bone Matrix and Hydroxyapatite
- Bone regeneration of the fluoridated hydroxyapatite and the bio-glass in the rabbit cranium defect model
- Bone healing capacity of the new fluoridated hydroxyapatite in the rabbit cranium defect