Yonsei Med J.
2012 Jul;53(4):715-722. 10.3349/ymj.2012.53.4.715.
The Clinical Characteristics of Steroid Responsive Nephrotic Syndrome of Children according to the Serum Immunoglobulin E Levels and Cytokines
- Affiliations
-
- 1Department of Pediatrics, Deajeon St. Mary's Hospital, The Catholic University of Korea, Daejeon, Korea.
- 2Department of Pediatrics, Chungnam National University School of Medicine, Daejeon, Korea. immlee@cnu.ac.kr
- KMID: 1716868
- DOI: http://doi.org/10.3349/ymj.2012.53.4.715
Abstract
- PURPOSE
The nephrotic syndrome (NS) is characterized by the favorable response to glucocorticoid therapy and the development of NS may be associated with dysfunctional immune systems. In order to investigate the serum immunoglobulin E (IgE) levels and cytokines activity in pediatric NS, the total of 32 steroid responsive NS patients and 5 healthy controls were enrolled in this study.
MATERIALS AND METHODS
All patients were divided into two groups according to the initial serum IgE levels, such as normal and high IgE group, and their clinical characteristics were evaluated. In addition, serum levels of interleukin (IL)-4, IL-5, IL-10 and transforming growth factor (TGF)-beta were compared and correlated with serum albumin, proteinuria by means of disease severity, and cytokines.
RESULTS
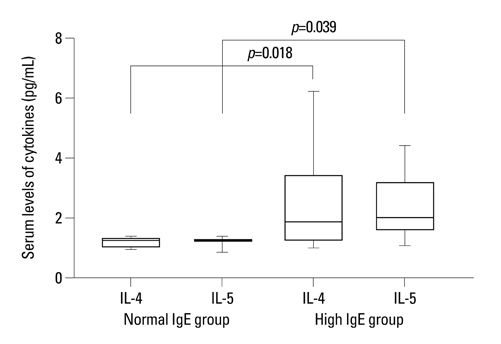
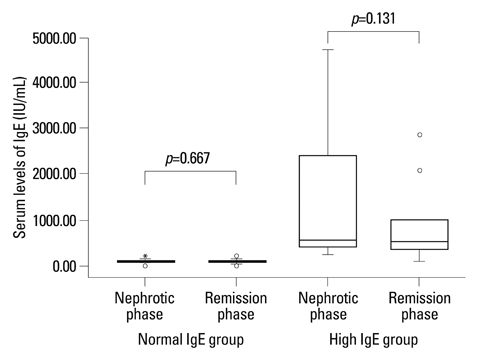
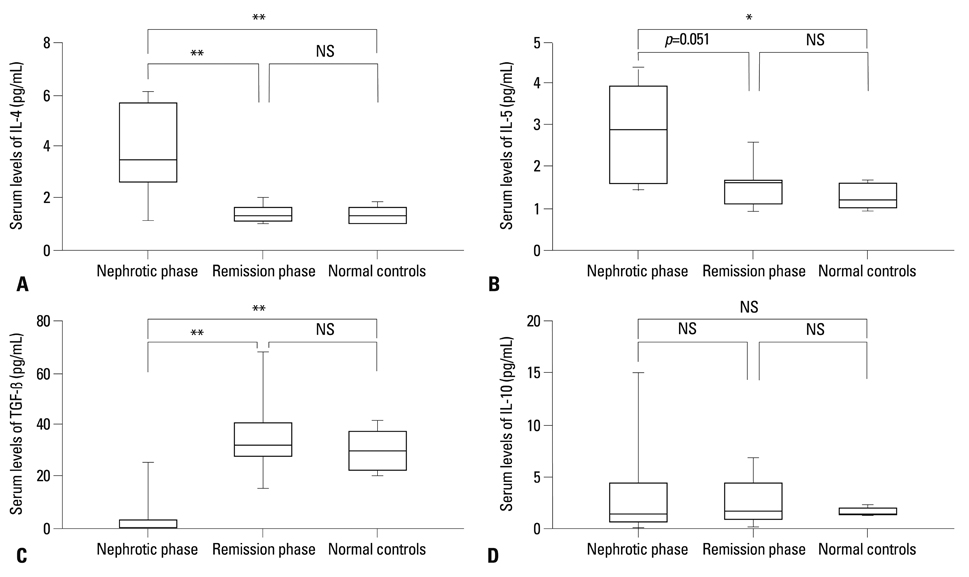
In the high IgE group, the higher comorbidity of allergic diseases and relapsing rate, the longer duration of steroid therapy before initial remission, and the higher serum IL-4 and IL-5 levels were found. In all patients, initially higher serum levels of IL-4 and IL-5 declined to normal levels after steroid therapy, whereas the serum IL-10 levels showed no significant difference between nephrotic phase (heavy proteinuria) and remission phase (no proteinuria) of NS. The serum TGF-beta levels of the nephrotic phase were significantly lower than those of remission phase or control group, and returned to normal control levels after steroid therapy.
CONCLUSION
This study indicates that initial IgE level is associated with steroid responsiveness and disease severity, and cytokine activities may also be related to the pathogenesis of pediatric steroid responsive NS.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Stepwise Treatment Using Corticosteroids Alone and in Combination with Cyclosporine in Korean Patients with Idiopathic Membranous Nephropathy
Dong Ho Shin, Mi Jung Lee, Hyung Jung Oh, Hyang Mo Koo, Fa Mee Doh, Hyoung Rae Kim, Jae Hyun Han, Jung Tak Park, Seung Hyeok Han, Kyu Hun Choi, Tae-Hyun Yoo, Shin-Wook Kang
Yonsei Med J. 2013;54(4):973-982. doi: 10.3349/ymj.2013.54.4.973.
Reference
-
1. Schachter AD. The pediatric nephrotic syndrome spectrum: clinical homogeneity and molecular heterogeneity. Pediatr Transplant. 2004. 8:344–348.
Article2. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981. 98:561–564.3. Hardwicke J, Soothill JF, Squire JR, Holti G. Nephrotic syndrome with pollen hypersensitivity. Lancet. 1959. 1:500–502.
Article4. Abdel-Hafez M, Shimada M, Lee PY, Johnson RJ, Garin EH. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis. 2009. 54:945–953.
Article5. Salsano ME, Graziano L, Luongo I, Pilla P, Giordano M, Lama G. Atopy in childhood idiopathic nephrotic syndrome. Acta Paediatr. 2007. 96:561–566.
Article6. van den Berg JG, Weening JJ. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci (Lond). 2004. 107:125–136.
Article7. Cheung W, Wei CL, Seah CC, Jordan SC, Yap HK. Atopy, serum IgE, and interleukin-13 in steroid-responsive nephrotic syndrome. Pediatr Nephrol. 2004. 19:627–632.
Article8. Grimbert P, Audard V, Remy P, Lang P, Sahali D. Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant. 2003. 18:245–248.
Article9. Matsumoto K, Kanmatsuse K. Transforming growth factor-beta1 inhibits vascular permeability factor release by T cells in normal subjects and in patients with minimal-change nephrotic syndrome. Nephron. 2001. 87:111–117.
Article10. Souto MF, Teixeira AL, Russo RC, Penido MG, Silveira KD, Teixeira MM, et al. Immune mediators in idiopathic nephrotic syndrome: evidence for a relation between interleukin 8 and proteinuria. Pediatr Res. 2008. 64:637–642.
Article11. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003. 362:629–639.
Article12. Le Berre L, Hervé C, Buzelin F, Usal C, Soulillou JP, Dantal J. Renal macrophage activation and Th2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 2005. 68:2079–2090.
Article13. Kanai T, Shiraishi H, Yamagata T, Ito T, Odaka J, Saito T, et al. Th2 cells predominate in idiopathic steroid-sensitive nephrotic syndrome. Clin Exp Nephrol. 2010. 14:578–583.
Article14. Araya C, Diaz L, Wasserfall C, Atkinson M, Mu W, Johnson R, et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2009. 24:1691–1698.
Article15. Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011. 139:314–320.
Article16. Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 1978. 13:159–165.17. Dodig S, Richter D, Benko B, Zivcić J, Raos M, Nogalo B, et al. Cut-off values for total serum immunoglobulin E between non-atopic and atopic children in north-west Croatia. Clin Chem Lab Med. 2006. 44:639–647.
Article18. Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974. 2:556–560.
Article19. Lin CY, Lee BH, Lin CC, Chen WP. A study of the relationship between childhood nephrotic syndrome and allergic diseases. Chest. 1990. 97:1408–1411.
Article20. Tain YL, Chen TY, Yang KD. Implication of serum IgE in childhood nephrotic syndrome. Pediatr Nephrol. 2003. 18:1211–1215.
Article21. Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000. 105(2 Pt 2):S547–S558.
Article22. Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl. 2005. S62–S67.
Article23. Fuke Y, Endo M, Ohsawa I, Satomura A, Hidaka M, Fujita T, et al. Implication of elevated serum IgE levels in minimal change nephrotic syndrome. Nephron. 2002. 91:769–770.
Article24. Finkelman FD, Katona IM, Urban JF Jr, Holmes J, Ohara J, Tung AS, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988. 141:2335–2341.25. Van Den Berg JG, Aten J, Chand MA, Claessen N, Dijkink L, Wijdenes J, et al. Interleukin-4 and interleukin-13 act on glomerular visceral epithelial cells. J Am Soc Nephrol. 2000. 11:413–422.
Article26. Daniel V, Trautmann Y, Konrad M, Nayir A, Schärer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997. 47:289–297.27. Ohtomo Y, Fujinaga S, Hattori M. Suplatast tosilate dimethylsulfonium treatment for steroid-dependent nephrotic syndrome. Pediatr Int. 2005. 47:230–231.
Article28. Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat Rev Immunol. 2009. 9:883–889.
Article29. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006. 212:8–27.
Article30. Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, et al. CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol. 2005. 16:1360–1370.
Article31. Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damião MJ, Cenedeze MA, et al. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009. 21:50–55.
Article32. Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, et al. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006. 17:2731–2741.
Article33. Shao XS, Yang XQ, Zhao XD, Li Q, Xie YY, Wang XG, et al. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr Nephrol. 2009. 24:1683–1690.
Article34. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998. 16:137–161.35. Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med. 2006. 57:365–380.
Article36. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006. 69:213–217.
Article37. Reidy K, Kaskel FJ. Pathophysiology of focal segmental glomerulosclerosis. Pediatr Nephrol. 2007. 22:350–354.
Article38. Gekle M, Knaus P, Nielsen R, Mildenberger S, Freudinger R, Wohlfarth V, et al. Transforming growth factor-beta1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol. 2003. 552(Pt 2):471–481.
Article39. Diwakar R, Pearson AL, Colville-Nash P, Brunskill NJ, Dockrell ME. The role played by endocytosis in albumin-induced secretion of TGF-beta1 by proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2007. 292:F1464–F1470.40. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003. 198:1875–1886.
Article41. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007. 317:256–260.
Article42. Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007. 8:950–957.
Article43. Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 2001. 10:51–59.
Article44. Mottet C, Golshayan D. CD4+CD25+Foxp3+ regulatory T cells: from basic research to potential therapeutic use. Swiss Med Wkly. 2007. 137:625–634.45. Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, et al. Immune dysregulation in TGF-beta 1-deficient mice. J Immunol. 1994. 153:1936–1946.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Serum Cytokines in Nephrotic Syndrome
- A Case of Kimura's Disease Occurring During Remission of Steroid-responsive Nephrotic Syndrome
- Steroid responsive nephrotic syndrome in mesangial IgA nephropathy
- A Case of Levamisole Treatment for Kimura's Disease-Associated Nephrotic Syndrome
- Clinicopathologic Characteristics of IgA Nephropathy with Steroid-responsive Nephrotic Syndrome