J Korean Med Sci.
2010 Aug;25(8):1197-1204. 10.3346/jkms.2010.25.8.1197.
Vaccination with a Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Cervical Cancer Vaccine in Korean Girls Aged 10-14 Years
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Hanyang University, Seoul, Korea. kimkt@hanyang.ac.kr
- 2Department of Obstetrics and Gynecology, Gangnamg Severance Hospital, Yonsei University, College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Keimyung University, Dongsan Medical Center, Daegu, Korea.
- 4Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, College of Medicine, Seoul National University, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Schools of Medicine, Sungkyunkwan University, Seoul, Korea.
- 6Department of Obstetrics and Gynecology, College of Medicine, University of Ulsan, Ulsan, Korea.
- 7Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 8Department of Obstetrics and Gynecology, College of Medicine, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 1714043
- DOI: http://doi.org/10.3346/jkms.2010.25.8.1197
Abstract
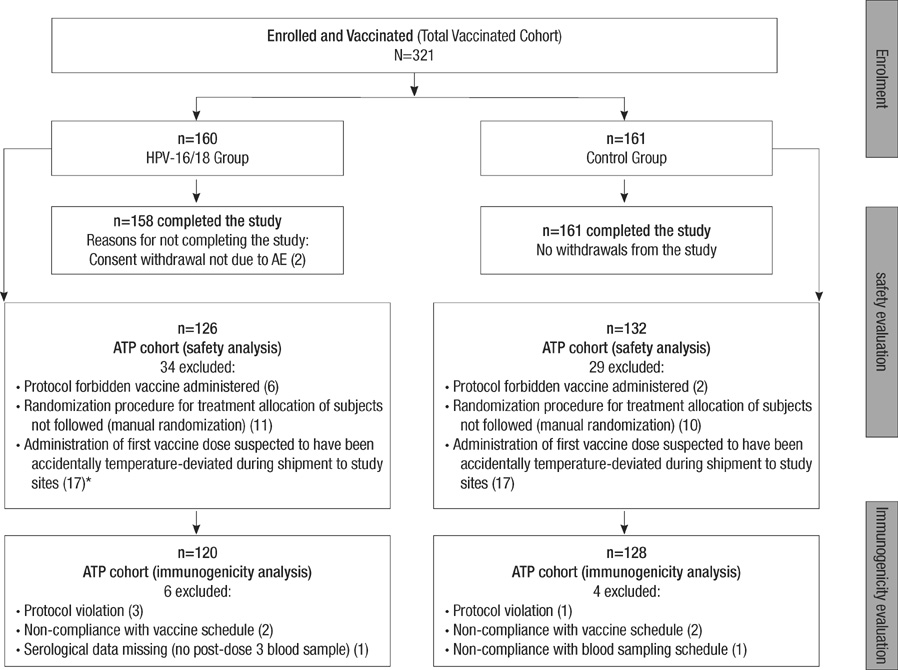
- The human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine has been demonstrated to be highly efficacious and immunogenic with a favorable safety profile. This study assessed the immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Korean girls aged 10-14 yr. This multi-center, observer-blind trial randomly assigned 321 healthy girls to receive three doses (0, 1, 6-month schedule) of HPV-16/18 AS04-adjuvanted vaccine or hepatitis A vaccine. Immunogenicity against vaccine antigens was assessed one month post-Dose 3. Solicited and unsolicited adverse events (AEs) and serious AEs (SAEs) were recorded. In the according-to-protocol analysis, all initially seronegative subjects vaccinated with the HPV-16/18 AS04-adjuvanted vaccine had seroconverted at Month 7, with a peak geometric mean titer (GMT) that was 600-fold higher than the natural infection titer of 29.8 EU/mL for HPV-16 and a peak GMT that was 400-fold higher than the natural infection titer of 22.6 EU/mL for HPV-18. The vaccine was well tolerated with no increase in reactogenicity with subsequent doses and no reports of vaccine-related SAEs. In conclusion, the HPV-16/18 AS04-adjuvanted vaccine is shown to be highly immunogenic and generally well-tolerated in Korean girls aged 10-14 yr.
Keyword
MeSH Terms
-
Adjuvants, Immunologic/administration & dosage
Adolescent
Aluminum Hydroxide/administration & dosage
Antibodies, Viral/analysis
Child
Female
Hepatitis A/immunology
Hepatitis A Vaccines/administration & dosage/adverse effects/immunology
Humans
Lipid A/administration & dosage/analogs & derivatives
Papillomavirus Infections/*prevention & control
Papillomavirus Vaccines/administration & dosage/adverse effects/*immunology
Republic of Korea
Seroepidemiologic Studies
Uterine Cervical Neoplasms/*prevention & control
Figure
Cited by 1 articles
-
Systemic Review for Efficacy of Human Papillomavirus Vaccines
Ho Sun Park
J Bacteriol Virol. 2011;41(4):313-318. doi: 10.4167/jbv.2011.41.4.313.
Reference
-
1. Castellsague X, de Sanjose S, Aguado T, Louie KS, Bruni L, Munoz J, Diaz M, Irwin K, Gacic M, Beauvais O, Albero G, Ferrer E, Byrne S, Bosch FX. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Vaccine. 2007. 25:Suppl 3. C1–C26.2. Ministry of Health and Welfare, Korea. 2002 Annual Report of Korea Central Cancer Registry. 2003. Seoul, South Korea: Ministry of Health and Welfare.3. Ferlay J, Bray F, Pisani P, Parkin DM. Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0. GLOBOCAN 2002. 2004. Lyon: IARC Press;Available at http://www-dep.iarc.fr/.4. Shin HR, Park S, Hwang SY, Kim JE, Jung KW, Won YJ, Hwang SS, Yim SH, Choi KS, Park EC, Park SY, Kim JW, Lee HP. Trends in cervical cancer mortality in Korea 1993-2002: Corrected mortality using national death certification data and national cancer incidence data. Int J Cancer. 2008. 122:393–397.
Article5. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, Lee HP. Members for Gynecologic Oncology Committee of Korean Society of Obstetrics and Gynecology. Cervical cancer incidence and survival in Korea 1993-2002. Int J Gynecol Cancer. 2006. 16:1833–1838.6. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007. 370:890–907.
Article7. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006. 24:Suppl 3. S3/42–S3/51.
Article8. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, Kim YT, Ng TL, Bock HL, Park JS. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008. 18:788–794.9. Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006. 98:303–315.10. Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005. 32:Suppl 1. S16–S24.
Article11. de Sanjosé S, Diaz M, Castellsague X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a metaanalysis. Lancet Infect Dis. 2007. 7:453–459.
Article12. Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Wacholder S, Tarone R, Burk RD. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005. 191:1808–1816.
Article13. Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen TE, Fortenberry JD. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005. 191:182–192.
Article14. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, De Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomized controlled trial. Lancet. 2007. 369:2161–2170.15. Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Perona P, Poncelet S, Zahaf T, Hardt K, Descamps D, Dubin G. HPV Study Group for Adult Women. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009. 27:581–587.
Article16. Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, Barbier S, Blatter MM, Chambers C, Ferris D, Gall SA, Guerra FA, Harper DM, Hedrick JA, Henry DC, Korn AP, Kroll R, Moscicki AB, Rosenfeld WD, Sullivan BJ, Thoming CS, Tyring SK, Wheeler CM, Dubin G, Schuind A, Zahaf T, Greenacre M, Sgriobhadair A. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009. 374:1975–1985.17. Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, Dubin G. HPV Vaccine Adolescent Study Investigators Network. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007. 40:564–571.
Article18. Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, Martin MT, Dubin G, Wettendorff MA. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006. 24:5937–5949.
Article19. Korean Center for Disease Control and Prevention. Second online survey for the health behavioral factors among adolescents in Korea in 2006. 2006. Seoul: Korea Center for Disease Control and Prevention.20. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, Jung KY, van Doorn LJ, Quint W. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004. 190:468–476.
Article21. Oh JK, Ju YH, Franceschi S, Quint W, Shin HR. Acquisition of new infection and clearance of type-specific human papillomavirus infections in female students in Busan, South Korea: a follow-up study. BMC Infect Dis. 2008. 8:13.
Article22. Konno R, Shin HR, Kim YT, Song YS, Sasagawa T, Inoue M, Park JS. Human papillomavirus infection and cervical cancer prevention in Japan and Korea. Vaccine. 2008. 26:Suppl 12. M30–M42.
Article23. Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Greenacre M. HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet. 2009. 374:301–314.24. Schiller JT, Davies P. Delivering on the promise: HPV vaccines and cervical cancer. Nat Rev Microbiol. 2004. 2:343–347.
Article25. Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, Grandi PD. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003. 95:1128–1137.
Article26. Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: Improving upon nature. Gynecol Oncol. 2008. 110:3 Suppl 1. S1–S10.
Article27. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, de Carvalho NS, Roteli-Martins CM, Teixeira J, Blatter MM, Korn AP, Quint W, Dubin G. GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004. 364:1757–1765.
Article28. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 2006. 367:1247–1255.29. Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, Dubin G. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009. 5:332–340.
Article30. Stanley M. Prophylactic HPV vaccines. J Clin Pathol. 2007. 60:961–965.
Article