J Gynecol Oncol.
2011 Jun;22(2):67-75. 10.3802/jgo.2011.22.2.67.
Human papillomavirus 16/18 AS04-adjuvanted cervical cancer vaccine: immunogenicity and safety in 15-25 years old healthy Korean women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, WCU Biomodulation in Major, Cancer Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 6GlaxoSmithKline Biologicals, Wavre, Belgium.
- 7Department of Obstetrics and Gynecology, Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea. jspark@catholic.ac.kr
- KMID: 2288569
- DOI: http://doi.org/10.3802/jgo.2011.22.2.67
Abstract
OBJECTIVE
The study assessed the immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Korean women aged 15-25 years.
METHODS
Phase IIIB, double-blind, randomised (2:1), multi-centre trial was conducted in Korea from June 2007 to March 2008. The study enrolled 225 women in the HPV (N=149) and placebo (N=76) groups who received three doses of HPV-16/18 AS04-adjuvanted vaccine or placebo (aluminium hydroxide) administered intramuscularly at 0, 1, and 6 months and were followed until one month post-dose 3. Serum samples were collected pre-vaccination and one month post-dose 3. Safety and reactogenicity data were collected throughout.
RESULTS
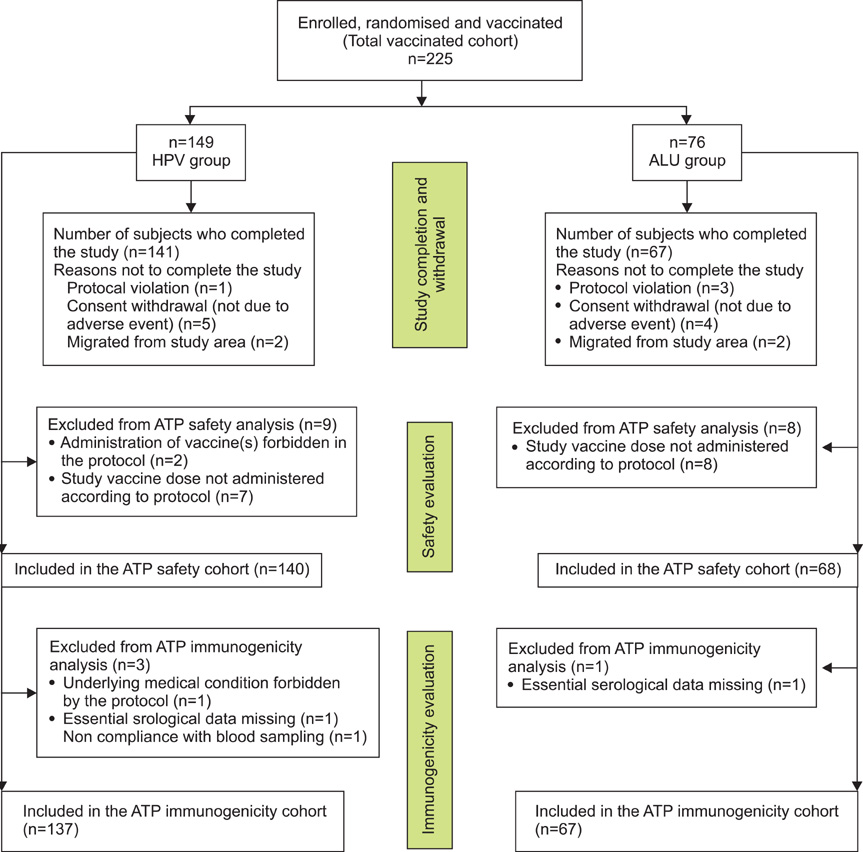
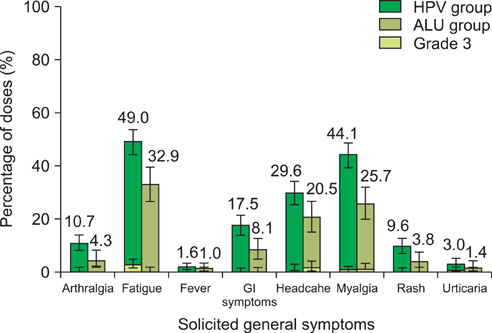
In this trial, 208 women completed the study (141 in HPV group; 67 in placebo group). At month 7, all initially seronegative women had seroconverted for HPV-16 and HPV-18 antibodies with anti-HPV-16 and anti-HPV-18 geometric mean titres of 9,351.4 El.U/mL (95% CI, 8,145.5 to 10,735.8) and 4204.1 El.U/mL (95% CI, 3,626.5 to 4,873.6), respectively. Initially seropositive women showed similar increase in geometric mean titre levels. Compliance to the three dose vaccination course was 95.3% in HPV and 89.5% in placebo group. Solicited local (pain) and general (fatigue, myalgia or headache) symptoms were commonly reported in both groups. Three serious adverse events were reported (two in HPV group; one in placebo group), all unrelated to vaccination by the investigator; all recovered.
CONCLUSION
The HPV-16/18 AS04-adjuvanted vaccine was highly immunogenic with a clinically acceptable safety profile in Korean women. This study was in line with previous global studies in Europe, North America, and Brazil. (ClinicalTrials.gov number, NCT 00485732.)
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Systemic Review for Efficacy of Human Papillomavirus Vaccines
Ho Sun Park
J Bacteriol Virol. 2011;41(4):313-318. doi: 10.4167/jbv.2011.41.4.313.
Reference
-
1. American Cancer Society. Global cancer facts and figures. 2007. Atlanta: American Cancer Society.2. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009. 20:1–7.3. Lee MY, Cho CH, Kwon SH, Song DK, Chung SW, Kang HO, et al. Annual report of gynecologic cancer registry program in Korean for 2002 (Jan. 1st, 2002 - Dec. 31st, 2002). Korean J Obstet Gynecol. 2004. 47:1029–1070.4. Shin HR, Park S, Hwang SY, Kim JE, Jung KW, Won YJ, et al. Trends in cervical cancer mortality in Korea 1993-2002: corrected mortality using national death certification data and national cancer incidence data. Int J Cancer. 2008. 122:393–397.5. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, editors. Cancer incidence in five continents. IARC Scientific Publications No. 160. 2007. Lyon: IARC.6. Bosch FX, de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers. 2007. 23:213–227.7. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007. 370:890–907.8. Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004. 111:278–285.9. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007. 121:621–632.10. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008. 18:788–794.11. Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec. 2009. 84:118–131.12. WHO grants prequalification to Cervarix: GSK's vaccine to help combat cervical cancer in developing nations [Internet]. c2001-2011. cited 2011 Jun 14. Middlessex: GlaxoSmithKline plc.;Available from: http://www.gsk.com/media/pressreleases/2009/2009_pressrelease_10072.htm.13. Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet. 2009. 374:301–314.14. Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine. 2008. 26:6630–6638.15. Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009. 5:332–340.16. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007. 369:2161–2170.17. Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007. 40:564–571.18. Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix]. Drugs. 2008. 68:359–372.19. Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, et al. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomized placebo-controlled trial up to 6.4 years. Lancet. 2009. 374:1975–1985.20. Stanley M. HPV vaccines: where are we now? J Fam Plann Reprod Health Care. 2007. 33:227–229.21. Cervarix, GSK's cervical cancer vaccine, wins tender for Dutch national immunisation programme [Internet]. c2001-2011. cited 2011 Jun 14. Middlessex: GlaxoSmithKline plc.;Available from: http://www.gsk.com/media/pressreleases/2008/2008_pressrelease_10123.htm.22. Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008. 4:425–434.23. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004. 364:1757–1765.24. Freeman HP, Wingrove BK. Excess cervical cancer mortality: a marker for low access to health care in poor communities. 2005. Rockville, MD: National Cancer Institute.25. Han CH, Cho HJ, Lee SJ, Bae JH, Bae SN, Namkoong SE, et al. The increasing frequency of cervical cancer in Korean women under 35. Cancer Res Treat. 2008. 40:1–5.26. Medina DM, Valencia A, de Velasquez A, Huang LM, Prymula R, Garcia-Sicilia J, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: a randomized, controlled trial in adolescent girls. J Adolesc Health. 2010. 46:414–421.27. Kim YJ, Kim KT, Kim JH, Cha SD, Kim JW, Bae DS, et al. Vaccination with a human papillomavirus (HPV)-16/18 AS04-adjuvanted cervical cancer vaccine in Korean girls aged 10-14 years. J Korean Med Sci. 2010. 25:1197–1204.28. Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000. 181:1911–1919.29. Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006. 24:5937–5949.30. Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009. 5:705–719.31. Oh JK, Ju YH, Franceschi S, Quint W, Shin HR. Acquisition of new infection and clearance of type-specific human papillomavirus infections in female students in Busan, South Korea: a follow-up study. BMC Infect Dis. 2008. 8:13.32. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004. 190:468–476.33. Jun JK, Choi KS, Jung KW, Lee HY, Gapstur SM, Park EC, et al. Effectiveness of an organized cervical cancer screening program in Korea: results from a cohort study. Int J Cancer. 2009. 124:188–193.34. Jang SN, Cho SI, Hwang SS, Jung-Choi K, Im SY, Lee JA, et al. Trend of socioeconomic inequality in participation in cervical cancer screening among Korean women. J Prev Med Public Health. 2007. 40:505–511.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccination with a Human Papillomavirus (HPV)-16/18 AS04-Adjuvanted Cervical Cancer Vaccine in Korean Girls Aged 10-14 Years
- Cervical Cancer and Human Papillomavirus Vaccines
- Efficacy of human papillomavirus vaccines including cross protection : A review of recent evidence
- Human Papillomavirus Vaccine
- Human Papillomavirus Vaccine