J Korean Med Sci.
2010 May;25(5):804-808. 10.3346/jkms.2010.25.5.804.
Loss of Y Chromosome in the Malignant Peripheral Nerve Sheet Tumor of a Patient with Neurofibromatosis Type 1
- Affiliations
-
- 1Department of Medical Genetics, School of Medicine, Ajou University, Suwon, Korea. genetics@kornet.net
- 2MG MED, Inc., Seoul, Korea.
- KMID: 1713971
- DOI: http://doi.org/10.3346/jkms.2010.25.5.804
Abstract
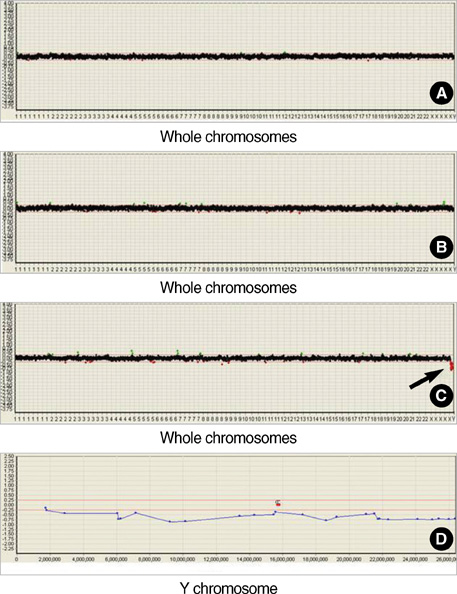
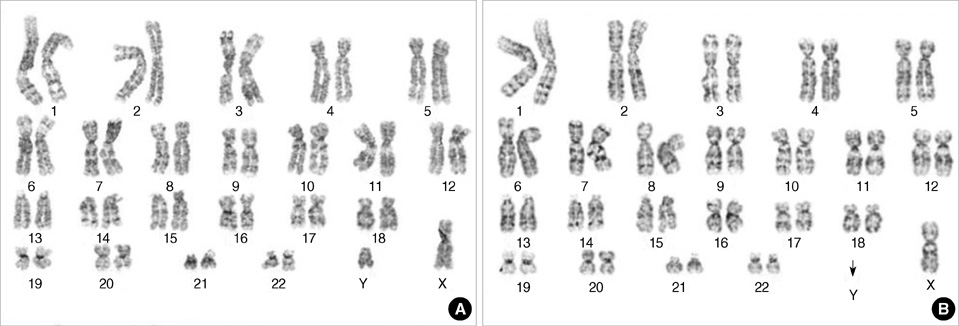
- Neurofibromatosis type 1 (NF1) is one of the most commonly inherited autosomal dominant disorders. In order to determine whether genomic alterations and/or chromosomal aberrations involved in the malignant progression of NF1 were present in a Korean patient with NF1, molecular and cytogenetic analyses were performed on the pathologically normal, benign, and malignant tissues and primary cells cultured from those tissues of the patient. The comparative genomic hybridization (CGH) array revealed a Y chromosome loss in the malignant peripheral nerve sheet tumor (MPNST) tissue. G-banding analysis of 50 metaphase cells showed normal chromosomal patterns in the histopathologically normal and benign cultured cells, but a mosaic Y chromosome loss in the malignant cells. The final karyotype for the malignant cells from MPNST tissue was 45,X,-Y[28]/46,XY[22]. The data suggest that the somatic Y chromosome loss may be involved in the transformation of benign tumors to MPNSTs.
Keyword
MeSH Terms
Figure
Reference
-
1. Savar A, Cestari DM. Neurofibromatosis type I: genetics and clinical manifestations. Semin Ophthalmol. 2008. 23:45–51.
Article2. Huson SM. Huson SM, Hughes RAC, editors. Neurofibromatosis 1: a clinical and genetic overview. The neurofibromatosis. 1994. London: Chapman and Hall Medical;160–203.3. Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002. 39:311–314.
Article4. Brunetti-Pierri N, Grange DK, Ou Z, Peiffer DA, Peacock SK, Cooper ML, Eng PA, Lalani SR, Chinault AC, Gunderson KL, Craigen WJ, Cheung SW. Characterization of de novo microdeletions involving 17q11.2q12 identified through chromosomal comparative genomic hybridization. Clin Genet. 2007. 72:411–419.
Article5. Legius E, Marchuk DA, Collins FS, Glover TW. Somatic deletion of the neurofibromatosis type 1 gene in a neurofibrosarcoma supports a tumour suppressor gene hypothesis. Nat Genet. 1993. 3:122–126.
Article6. Jeong SY, Han JH, Park YY, Kim HJ. Identification of differentially expressed genes related to NF1-associated malignant transformation from a patient with neurofibromatosis type 1. Genes and Genomics. 2008. 30:407–418.7. Koga T, Iwasaki H, Ishiguro M, Matsuzaki A, Kikuchi M. Frequent genomic imbalances in chromosomes 17, 19, and 22q in peripheral nerve sheath tumours detected by comparative genomic hybridization analysis. J Pathol. 2002. 197:98–107.
Article8. Yano S, Matsuyama H, Matsuda K, Matsumoto H, Yoshihiro S, Naito K. Accuracy of an array comparative genomic hybridization (CGH) technique in detecting DNA copy number aberrations: comparison with conventional CGH and loss of heterozygosity analysis in prostate cancer. Cancer Genet Cytogenet. 2004. 150:122–127.
Article9. Serra E, Puig S, Otero D, Gaona A, Kruyer H, Ars E, Estivill X, Lázaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997. 61:512–519.10. Upadhyaya M, Kluwe L, Spurlock G, Monem B, Majounie E, Mantripragada K, Ruggieri M, Chuzhanova N, Evans DG, Ferner R, Thomas N, Guha A, Mautner V. Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs). Hum Mutat. 2008. 29:74–82.11. Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008. 56:1590–1605.
Article12. Lothe RA, Karhu R, Mandahl N, Mertens F, Sauter G, Heim S, Borresen-Dale AL, Kallioneimi OP. Gain of 17q24-qter detected by comparative genomic hybridization in malignant tumors from patients with von Recklinghausen's neurofibromatosis. Cancer Res. 1996. 56:4778–4781.13. Schmidt H, Taubert H, Meye A, Würl P, Bache M, Bartel F, Holzhausen HJ, Hinze R. Gains in chromosomes 7, 8q, 15q and 17q are characteristic changes in malignant but not in benign peripheral nerve sheath tumors from patients with Recklinghausen's disease. Cancer Lett. 2000. 155:181–190.
Article14. Bartsch O, Vlcková Z, Erdogan F, Ullmann R, Novotná D, Spiegel M, Beyer V, Haaf T, Zechner U, Seemanová E. Two independent chromosomal rearrangements, a very small (550 kb) duplication of the 7q subtelomeric region and an atypical 17q11.2 (NF1) microdeletion, in a girl with neurofibromatosis. Cytogenet Genome Res. 2007. 119:158–164.15. Wong AK, Fang B, Zhang L, Guo X, Lee S, Schreck R. Loss of the Y chromosome: an age-related or clonal phenomenon in acute myelogenous leukemia/myelodysplastic syndrome? Arch Pathol Lab Med. 2008. 132:1329–1332.
Article16. Wallrapp C, Hahnel S, Boeck W, Soder A, Mincheva A, Lichter P, Leder G, Gansauge F, Sorio C, Scarpa A, Gress TM. Loss of the Y chromosome is a frequent chromosomal imbalance in pancreatic cancer and allows differentiation to chronic pancreatitis. Int J Cancer. 2001. 91:340–344.
Article17. Kujawski M, Jarmuz M, Rydzanicz M, Szukala K, Wierzbicka M, Grenman R, Golusinski W, Szyfter K. Frequent chromosome Y loss in primary, second primary and metastatic squamous cell carcinomas of the head and neck region. Cancer Lett. 2004. 208:95–101.
Article18. Wiktor A, Rybicki BA, Piao ZS, Shurafa M, Barthel B, Maeda K, Van Dyke DL. Clinical significance of Y chromosome loss in hematologic disease. Genes Chromosomes Cancer. 2000. 27:11–16.
Article19. Park SJ, Jeong SY, Kim HJ. Y chromosome loss and other genomic alterations in hepatocellular carcinoma cell lines analyzed by CGH and CGH array. Cancer Genet Cytogenet. 2006. 166:56–64.
Article20. Stevenson DA, Birch PH, Friedman JM, Viskochil DH, Balestrazzi P, Boni S, Buske A, Korf BR, Niimura M, Pivnick EK, Schorry EK, Short MP, Tenconi R, Tonsgard JH, Carey JC. Descriptive analysis of tibial pseudarthrosis in patients with neurofibromatosis 1. Am J Med Genet. 1999. 84:413–419.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Malignant Peripheral Nerve Sheath Tumor of the Cauda Equina in Type I Neurofibromatosis: Case Report
- A Case of Malignant Peripheral Nerve Sheath Tumor of the Neck Associated with Neurofibromatosis Type I
- Malignant Peripheral Nerve Sheath Tumor Arising from Neurofibromatosis
- An intrathoracic malignant peripheral nerve sheath tumor in a neurofibromatosis type 1 patient
- A Case of Malignant Peripheral Nerve Sheath Tumor in Neurofibromatosis