J Korean Med Sci.
2010 Jan;25(1):61-66. 10.3346/jkms.2010.25.1.61.
Examination of Geographical, Clinical and Intrahost Variations in the 3' Repeat Region of CagA Gene in Helicobacter pylori
- Affiliations
-
- 1Department of Gastroenterology, Kyungpook National University, Daegu, Korea. skim@knu.ac.kr
- KMID: 1713833
- DOI: http://doi.org/10.3346/jkms.2010.25.1.61
Abstract
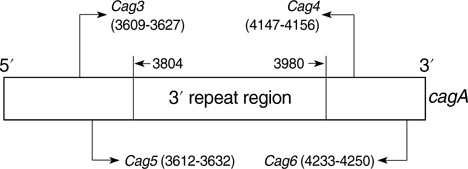
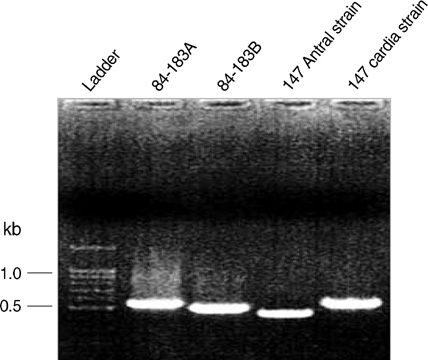
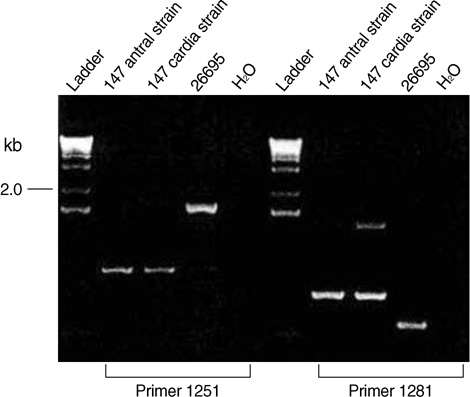
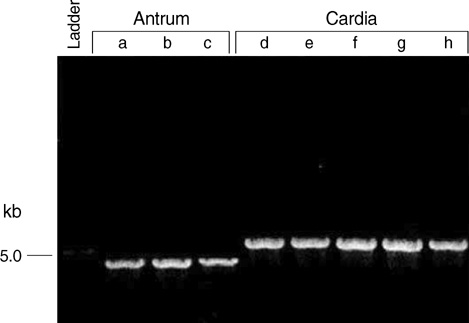
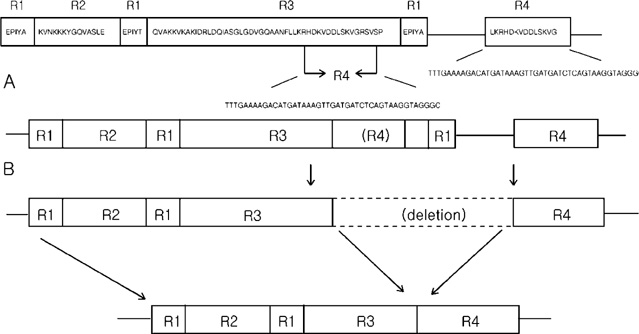
- The size variation of the cytoxin-associated protein (cagA), which is dependent on the 3' repeat region (3'RR) of the cagA gene, is known to play a crucial role in the pathogenesis of Helicobacter pylori infection. The present study evaluated the relationship between the 3'RR variation and the geographic distribution, clinical manifestations, and locations of colonization in the stomach. We evaluated the 3'RR of H. pylori isolates from 78 patients with gastric cancer, peptic ulcer, and non-ulcer dyspepsia from Japan, Hong Kong, India, and the United States and assessed the variations of 3'RR according to the geographical and clinical characteristics. Sixty eight (87.2%) patients had the same 650 bp band without geographical differences. The frequency of polymorphisms in the 3'RR did not differ when compared to the clinical manifestations (P=0.868). The length of 3'RR did not differ by location of colonization. In conclusion, the 3'RR variation of cagA gene is not associated with the geographical and clinical characteristics of the patients studied.
Keyword
MeSH Terms
-
Amino Acid Sequence
Antigens, Bacterial/*genetics
Bacterial Proteins/*genetics
Dyspepsia/etiology
Helicobacter Infections/diagnosis
Helicobacter pylori/*genetics
Humans
Integration Host Factors
Molecular Sequence Data
Peptic Ulcer/etiology
Polymorphism, Genetic
Repetitive Sequences, Amino Acid
Repetitive Sequences, Nucleic Acid
Stomach Neoplasms/etiology
Antigens, Bacterial
Bacterial Proteins
Integration Host Factors
Figure
Reference
-
1. Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998. 28:37–53.
Article2. Atherton JC. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997. 40:701–703.
Article3. Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993. 90:5791–5795.
Article4. Cover TL, Dooley CP, Blaser MJ. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990. 58:603–610.
Article5. Soltermann A, Koetzer S, Eigenmann F, Komminoth P. Correlation of Helicobacter pylori virulence genotypes vacA and cagA with histological parameters of gastritis and patient's age. Mod Pathol. 2007. 20:878–883.
Article6. Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993. 61:1799–1809.
Article7. Umit H, Tezel A, Bukavaz S, Unsal G, Otkun M, Soylu AR, Tucer D, Bilgi S. The relationship between virulence factors of Helicobacter pylori and severity of gastritis in infected patients. Dig Dis Sci. 2009. 54:103–110.
Article8. Valmaseda Perez T, Gisbert JP, Pajares Garcia JM. Geographic differences and the role of cagA gene in gastroduodenal diseases associated with Helicobacter pylori infection. Rev Esp Enferm Dig. 2001. 93:471–480.9. Yamaoka Y, El-Zimaity HM, Gutierrez O, Figura N, Kim JG, Kodama T, Kashima K, Graham DY. Relationship between the cagA 3' repeat region of Helicobacter pylori, gastric histology, and susceptibility to low pH. Gastroenterology. 1999. 117:342–349.
Article10. Yamaoka Y, Kodama T, Kashima K, Graham DY, Sepulveda AR. Variants of the 3' region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998. 36:2258–2263.11. Maeda S, Kanai F, Ogura K, Yoshida H, Ikenoue T, Takahashi M, Kawabe T, Shiratori Y, Omata M. High seropositivity of anti-CagA antibody in Helicobacter pylori-infected patients irrelevant to peptic ulcers and normal mucosa in Japan. Dig Dis Sci. 1997. 42:1841–1847.12. Saruc M, Demir MA, Kucukmetin N, Kandiloglu AR, Akarca US, Yuceyar H. Histological and clinical predictive value of determination of tissue CagA status by PCR in Helicobacter pylori infected patients; results of the large population based study in western Turkey. Hepatogastroenterology. 2002. 49:878–881.13. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999. 37:2274–2279.
Article14. Akopyanz N, Bukanov NO, Westblom TU, Kresovich S, Berg DE. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992. 20:5137–5142.15. Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996. 93:14648–14653.
Article16. Tummuru MK, Sharma SA, Blaser MJ. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995. 18:867–876.
Article17. Weel JF, van der Hulst RW, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat GN, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996. 173:1171–1175.
Article18. Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995. 63:94–98.
Article19. Cover TL, Blaser MJ. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996. 41:85–117.20. Zhou J, Zhang J, Xu C, He L. CagA genotype and variants in Chinese Helicobacter pylori strains and relationship to gastroduodenal diseases. J Med Microbiol. 2004. 53:231–235.
Article21. Kidd M, Lastovica AJ, Atherton JC, Louw JA. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999. 45:499–502.
Article22. Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle PR, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998. 36:944–948.23. Karita M, Matsumoto S, Kamei T. The size of cagA based on repeat sequence has the responsibility of the location of Helicobacter pylori in the gastric mucus and the degree of gastric mucosal inflammation. Microbiol Immunol. 2003. 47:619–630.24. Choi KD, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. Analysis of the 3' variable region of the cagA gene of Helicobacter pylori isolated in Koreans. Dig Dis Sci. 2007. 52:960–966.
Article25. Go MF, Graham DY. Presence of the cagA gene in the majority of Helicobacter pylori strains is independent of whether the individual has duodenal ulcer or asymptomatic gastritis. Helicobacter. 1996. 1:107–111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of the Helicobacter pylori CagA Gene in Gastric Cancer Tissue of Koreans
- Histopathologic Analysis of Helicobacter pylori-associated Chronic Gastritis between cagA-positive and cagA-negative Strains
- Significance of cagA Gane in Gastric Biopsy Specimens of Normal Subject Without Gastroscopic Abnormality
- The Positive Rates of Helicobacter pylori cagA Gene in Gastric Biopsy Specimens of the Patients with Gastritis , Gastric Ulcer , Duodenal Ulcer , and Gastric Cancer and Comparison of the Degree of Gastritis
- Positivity of cagA and vacA Genes of Helicobacter pylori by PCR Assay of Gastric Biopsy Specimens and Gastric Inflammation in Children