Lab Anim Res.
2014 Jun;30(2):73-78. 10.5625/lar.2014.30.2.73.
Effects of anti-obesity drugs, phentermine and mahuang, on the behavioral patterns in Sprague-Dawley rat model
- Affiliations
-
- 1Laboratory of Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- 2Laboratory of Toxicology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 1707446
- DOI: http://doi.org/10.5625/lar.2014.30.2.73
Abstract
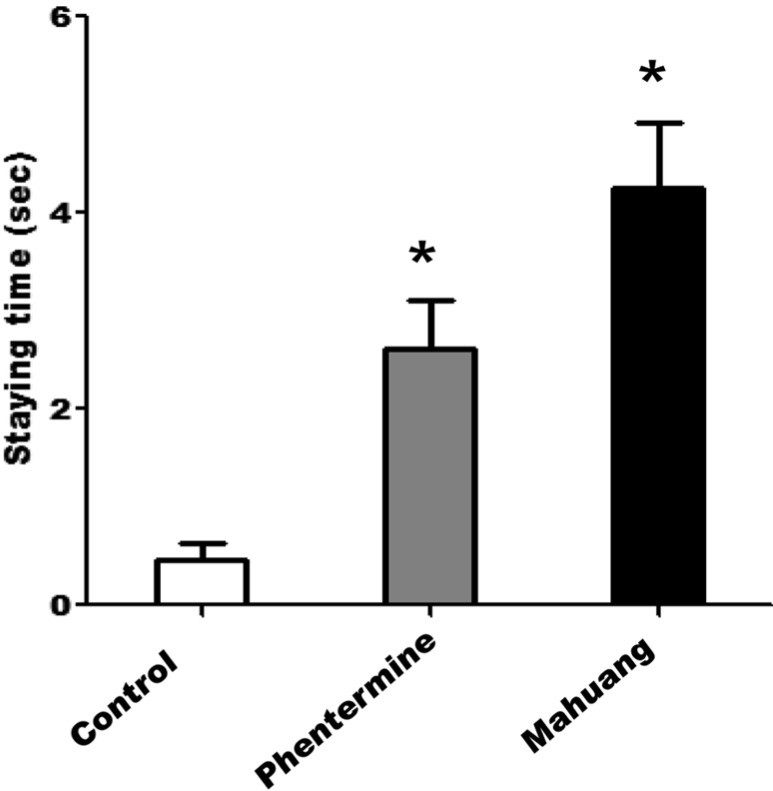
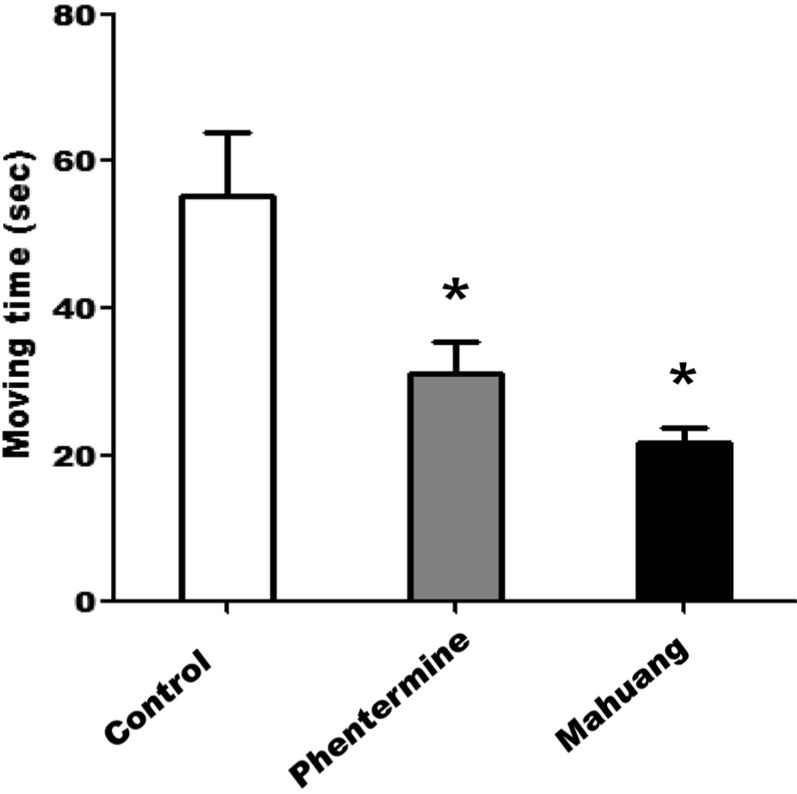
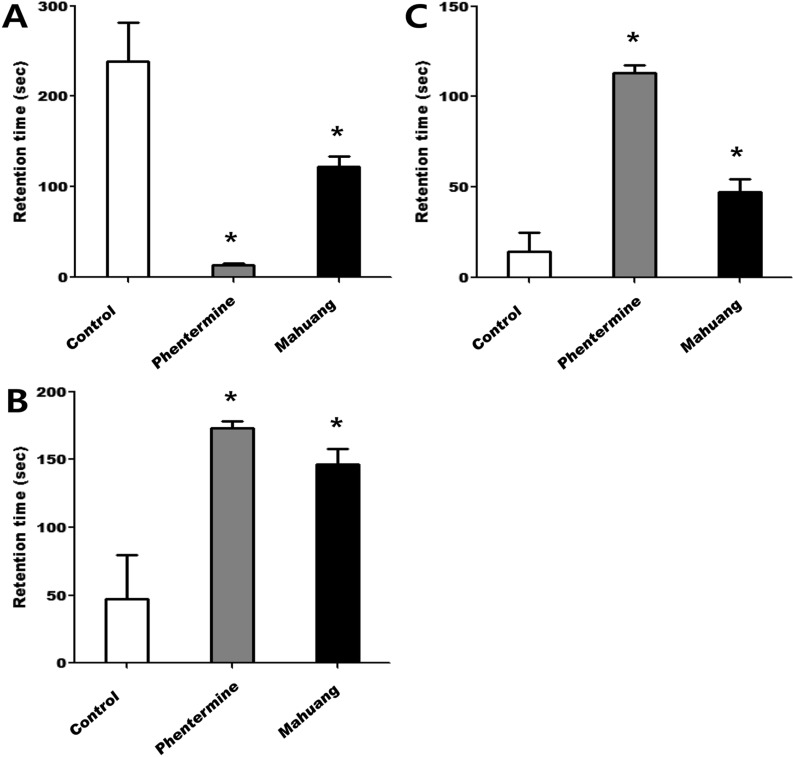
- According to WHO global estimates from 2008, more than 1.4 billion adults were overweight and among them, over 200 million men and 300 million women were obese. Although the main treatment modalities for overweight and obese individuals remain dieting and physical exercise, the synthetic anti-obesity medications have been increasingly used due to their perceived convenience. Generally, anti-obesity medications are classified as appetite suppressants or fat absorption blockers. In the present study, we examined the adverse side-effects in respect of behavior changes of phentermine and Ephedra sinica (mahuang) that are anti-obesity drugs currently distributed to domestic consumers. Phentermine is mainly classified as an anorexing agent and mahuang a thermogenic agent. Because phentermine and mahuang are considered to display effectiveness through the regulation of nerve system, their potential influences of on behavioral changes were examined employing animal experiments. From the results of experiments testing locomotor activity through the use of treadmill, rota-rod, and open field system, phentermine and mahuang were commonly revealed to induce behavioral changes of rats by reducing a motor ability, an ability to cope with an external stimulus, and a sense of balance or by augmenting wariness or excitement. These adverse effects of phenternime and mahuang in behavioral changes need to be identified in humans and anti-obesity medications such as phentermine and mahuang should be prescribed for only obesity where it is anticipated that the benefits of the treatment outweigh their potential risks.
Keyword
MeSH Terms
Figure
Reference
-
1. Bianco AC, McAninch EA. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. Lancet Diabetes Endocrinol. 2013; 1(3):250–258. PMID: 24622373.
Article2. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009; 53(21):1925–1932. PMID: 19460605.3. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001; 409(6818):307–312. PMID: 11201732.
Article4. Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond). 2005; 29(10):1168–1174. PMID: 15925949.
Article5. Kelley GA, Kelley KS. Effects of exercise in the treatment of overweight and obese children and adolescents: a systematic review of meta-analyses. J Obes. 2013; 2013:783103. PMID: 24455215.
Article6. Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006; 5(11):919–931. PMID: 17080028.
Article7. Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006; 29(4):277–302. PMID: 16569079.
Article8. Trigueros L, Peña S, Ugidos AV, Sayas-Barberá E, Pérez-Álvarez JA, Sendra E. Food ingredients as anti-obesity agents: a review. Crit Rev Food Sci Nutr. 2013; 53(9):929–942. PMID: 23768185.
Article9. McClendon KS, Riche DM, Uwaifo GI. Orlistat: current status in clinical therapeutics. Expert Opin Drug Saf. 2009; 8(6):727–744. PMID: 19998527.
Article10. Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008; 31(1):53–65. PMID: 18095746.11. Akbas F, Gasteyger C, Sjödin A, Astrup A, Larsen TM. A critical review of the cannabinoid receptor as a drug target for obesity management. Obes Rev. 2009; 10(1):58–67. PMID: 18721231.
Article12. Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa PI, Covel JA, Ren A, Webb RR, Beeley NR, Martin M, Morgan M, Espitia S, Saldana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008; 51(2):305–313. PMID: 18095642.
Article13. Shyh G, Cheng-Lai A. New antiobesity agents: lorcaserin (Belviq) and phentermine/topiramate ER (Qsymia). Cardiol Rev. 2014; 22(1):43–50. PMID: 24304809.14. Snow V, Barry P, Fitterman N, Qaseem A, Weiss K. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005; 142(7):525–531. PMID: 15809464.
Article15. Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J. 2012; 36(1):13–25. PMID: 22363917.
Article16. Abourashed EA, El-Alfy AT, Khan IA, Walker L. Ephedra in perspective--a current review. Phytother Res. 2003; 17(7):703–712. PMID: 12916063.17. Song MK, Um JY, Jang HJ, Lee BC. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp Ther Med. 2012; 3(4):707–712. PMID: 22969956.
Article18. Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagné J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA. 2003; 289(12):1537–1545. PMID: 12672771.
Article19. Shekelle P, Hardy M. Safety and efficacy of ephedra and ephedrine for enhancement of athletic performance, thermogenesis and the treatment of obesity. Phytomedicine. 2002; 9(1):78. PMID: 11924769.20. Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, Salim S. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav. 2014; 130:47–53. PMID: 24657739.
Article21. Yang G, Park D, Lee SH, Bae DK, Yang YH, Kyung J, Kim D, Choi EK, Hong JT, Jeong HS, Kim HJ, Jang SK, Joo SS, Kim YB. Neuroprotective Effects of a Butanol Fraction of Rosa hybrida Petals in a Middle Cerebral Artery Occlusion Model. Biomol Ther (Seoul). 2013; 21(6):454–461. PMID: 24404336.
Article22. Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010; 12(10):876–882. PMID: 20920040.
Article23. Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005; 142(7):532–546. PMID: 15809465.
Article24. Hackman RM, Havel PJ, Schwartz HJ, Rutledge JC, Watnik MR, Noceti EM, Stohs SJ, Stern JS, Keen CL. Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. Int J Obes (Lond). 2006; 30(10):1545–1556. PMID: 16552410.
Article