Ann Lab Med.
2013 Jul;33(4):233-241. 10.3343/alm.2013.33.4.233.
Is Prostate-Specific Antigen Effective for Population Screening of Prostate Cancer? A Systematic Review
- Affiliations
-
- 1Department of Health Technology Assessment Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea.
- 2Department of Oriental Gynecology, Bundang CHA Medical Center, CHA University, Seongnam, Korea.
- 3Department of Health Policy and Management, School of Public Health, Seoul National University, Seoul, Korea.
- 4Department of Laboratory Medicine and Genetics, Soonchunhyang University College of Medicine, Bucheon, Korea. youklee@gmail.com
- 5Department of Family Medicine, College of Medicine, Hallym University, Seoul, Korea.
- KMID: 1707229
- DOI: http://doi.org/10.3343/alm.2013.33.4.233
Abstract
- BACKGROUND
The effectiveness of prostate-specific antigen (PSA) for population screening has presented controversial results in large trials and prior reviews. We investigated the effectiveness of PSA population screening in a systematic review.
METHODS
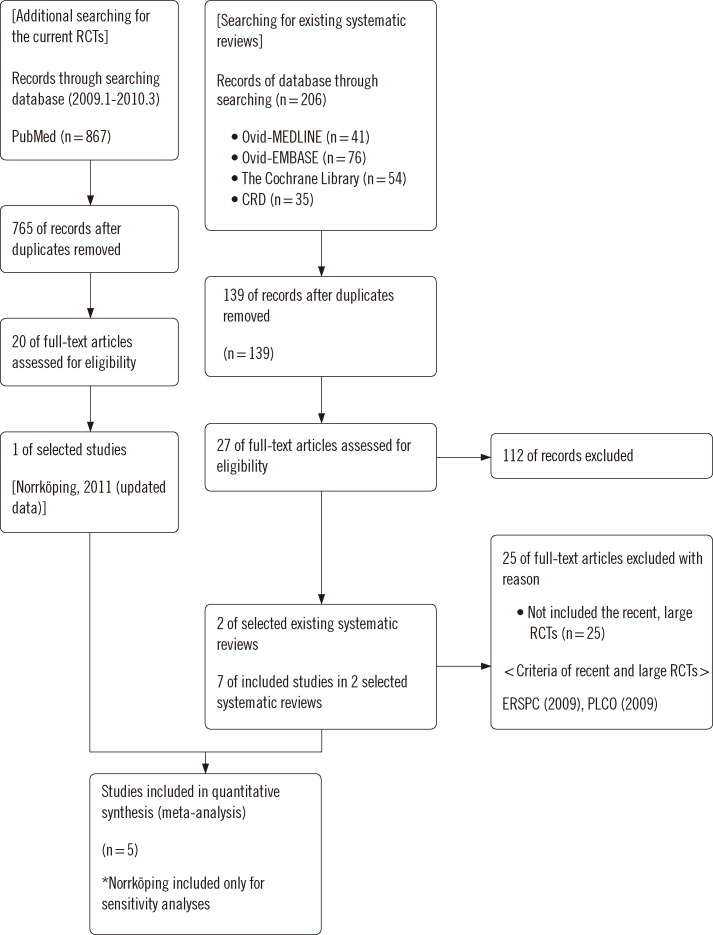
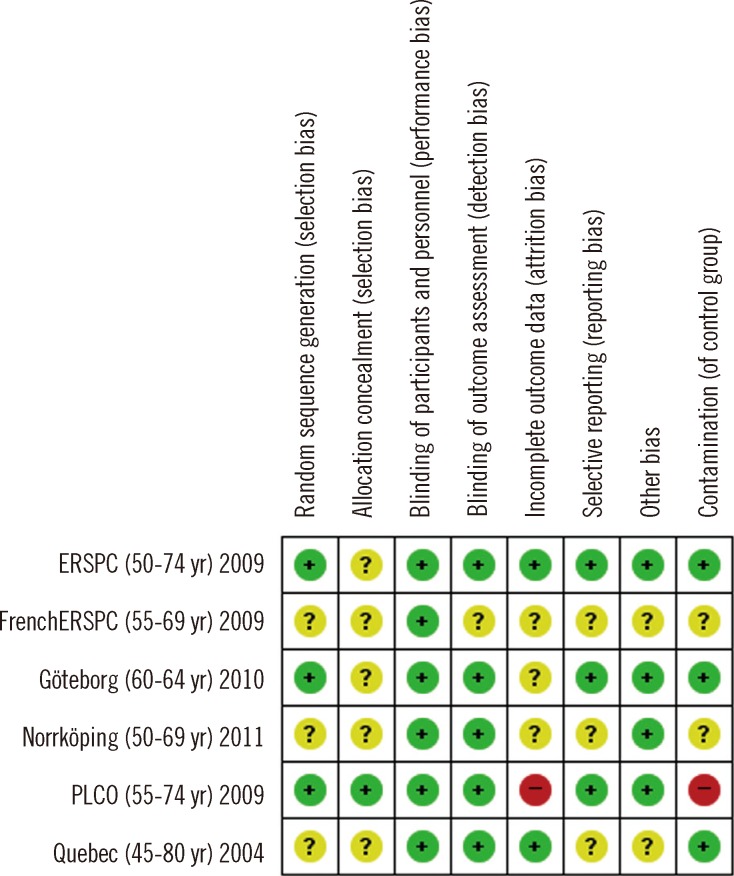
The study was conducted using existing systematic reviews. We searched Ovid MEDLINE, Embase, Cochrane library, and the major Korean databases. The quality of the systematic reviews was assessed by two reviewers independently using AMSTAR. Randomized controlled trials were assessed using the risk of bias tool in the Cochrane group. Meta-analyses were conducted using Review Manager. The level of evidence of each outcome was assessed using GRADE.
RESULTS
Prostate-cancer-specific mortality was not reduced based on similar prior reviews (relative risk [RR] 0.93; 95% confidence interval [CI], 0.81-1.07, P=0.31). The detection rate of stage 1 prostate cancer was not greater, with a RR of 1.67 (95% CI, 0.95-2.94) and high heterogeneity. The detection rate of all cancer stages in the screening group was high, with a RR of 1.45 (95% CI, 1.13-1.85). No difference in all-cause mortality was observed between the screening and control groups (RR, 0.99; 95% CI, 0.98-1.01, P=0.50). Prostate-cancer-specific mortality, all-cause mortality, and diagnosis of prostate cancer at stages 3-4 showed moderate levels of evidence.
CONCLUSIONS
Differently from prior studies, our review included updated Norrkoping data and assessed the sole effect of PSA testing for prostate cancer screening. PSA screening alone did not increase early stage prostate cancer detection and did not lower mortality.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917. PMID: 21351269.
Article2. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur Urol. 2012; 61:1079–1092. PMID: 22424666.
Article3. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009; 360:1310–1319. PMID: 19297565.
Article4. Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009; 360:1320–1328. PMID: 19297566.
Article5. Djulbegovic M, Beyth RJ, Neuberger MM, Stoffs TL, Vieweg J, Djulbegovic B, et al. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010; 341:c4543. PMID: 20843937.
Article6. Ilic D, O'Connor D, Green S, Wilt TJ. Screening for prostate cancer. Cochrane Database Syst Rev. 2010; 11.
Article7. Lumen N, Fonteyne V, De Meerleert G, Ost P, Villeirs G, Mottrie A, et al. Population screening for prostate cancer: an overview of available studies and meta-analysis. Int J Urol. 2012; 19:100–108. PMID: 22103653.
Article8. Romero FR, Romero AW, Brenny Filho T, Bark NM, Yamazaki DS, de Oliveira FC. Patients' perceptions of pain and discomfort during digital rectal exam for prostate cancer screening. Arch Esp Urol. 2008; 61:850–854. PMID: 18972927.
Article9. Hamashima C, Nakayama T, Sagawa M, Saito H, Sobue T. The Japanese guideline for prostate cancer screening. Jpn J Clin Oncol. 2009; 39:339–351. PMID: 19346535.
Article10. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011; 59:61–71. PMID: 21056534.
Article11. Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010; 60:70–98. PMID: 20200110.
Article12. Harvey P, Basuita A, Endersby D, Curtis B, Iacovidou A, Walker M. A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol. 2009; 9:14. PMID: 19744336.
Article13. Crawford ED, Abrahamsson PA. PSA-based screening for prostate cancer: how does it compare with other cancer screening tests? Eur Urol. 2008; 54:262–273. PMID: 18556114.
Article14. White CM, Ip S, McPheeters M, Cary TS, Chou R, Lohr KN, et al. Using existing systematic reviews to replace de novo processes in conducting comparative effectivenss reviews. Methods guide for comparative effectiveness reviews. Rockville: Agency for Healthcare Research and Quality;2009.15. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7:10. PMID: 17302989.
Article16. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and udertaking meta-analyses. In : Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (updated March 2011). The Cochrane Collaboration;2011.17. Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010; 11:725–732. PMID: 20598634.
Article18. Labrie F, Candas B, Cusan L, Gomez JL, Bélanger A, Brousseau G, et al. Screening decreases prostate cancer mortality: 11-year follow-up of the 1988 Quebec prospective randomized controlled trial. Prostate. 2004; 59:311–318. PMID: 15042607.
Article19. Jegu J, Tretarre B, Grosclaude P, Rebillard X, Bataille V, Malavaud B, et al. Results and participation factors to the European Randomized study of Screening for Prostate Cancer (ERSPC) with Prostate Specific Antigen: French departments of Tarn and Hérault. Prog Urol. 2009; 19:487–498. PMID: 19559380.20. Kjellman A, Akre O, Norming U, Törnblom M, Gustafsson O. 15-year followup of a population based prostate cancer screening study. J Urol. 2009; 181:1615–1621. PMID: 19233435.
Article21. Sandblom G, Varenhorst E, Löfman O, Rosell J, Carlsson P. Clinical consequences of screening for prostate cancer: 15 years follow-up of a randomised controlled trial in Sweden. Eur Urol. 2004; 46:717–723. PMID: 15548438.
Article22. Sandblom G, Varenhorst E, Rosell J, Löfman O, Carlsson P. Randomised prostate cancer screening trial: 20 year follow-up. BMJ. 2011; 342:d1539. PMID: 21454449.
Article23. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012; 366:981–990. PMID: 22417251.
Article24. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012; 104:125–132. PMID: 22228146.25. Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 157:120–134. PMID: 22801674.
Article26. Lee EH, Han MA, Lee HY, Jun JK, Choi KS, Park EC. Liver cancer screening in Korea: a report on the 2008 National Cancer Screening Programme. Asian Pac J Cancer Prev. 2010; 11:1305–1310. PMID: 21198282.27. Hahm MI, Choi KS, Lee HY, Jun JK, Oh D, Park EC. Who participates in the gastric cancer screening and on-time rescreening in the National Cancer Screening Program? A population-based study in Korea. Cancer Sci. 2011; 102:2241–2247. PMID: 21895871.
Article28. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010; 102:605–613. PMID: 20413742.
Article29. Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009; 101:374–383. PMID: 19276453.
Article30. Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). Eur Urol. 2009; 56:584–591. PMID: 19660851.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening for Prostatic Cancers in Korean
- What Are Some New Developments in Prostate Cancer Diagnosis?
- The Diagnostic Value of Prostate-specific Antigen and the of Routine Laboratory Examination for Early Detection
- The prostate specific antigen in detection of the prostate cancer
- Prostate-Specific Antigen-Based Prostate Cancer Screening: One for All or Individualized for Each Race? – A Narrative Review