Tuberc Respir Dis.
2006 Jun;60(6):638-644. 10.4046/trd.2006.60.6.638.
Association between the Human Surfactant Protein-A(SP-A) Gene Locus and Chronic Obstructive Pulmonary Disease in Korean Population
- Affiliations
-
- 1Department of Internal Medicine, Soonchunhyang University Collage of Medicine, Cheonan, Korea. juokna@schch.co.kr
- 2Department of Pediatrics Soonchunhyang University Collage of Medicine, Cheonan, Korea.
- KMID: 1630802
- DOI: http://doi.org/10.4046/trd.2006.60.6.638
Abstract
-
BACKGROUNDS: This study investigated whether or not a polymorphism in the gene encoding the surfactant protein A(SP-A) has any bearing on the individual susceptibility to the development of chronic obstructive pulmonary disease(COPD) in a genetically homogenous Korean population.
METHODS
The genotypes of 19 COPD patients and 20 healthy neonates as controls were tested using a polymerase chain reaction followed by restriction fragment length polymorphism analysis for the SP-A gene.
RESULTS
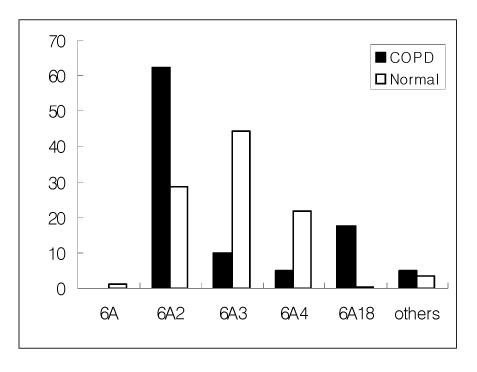
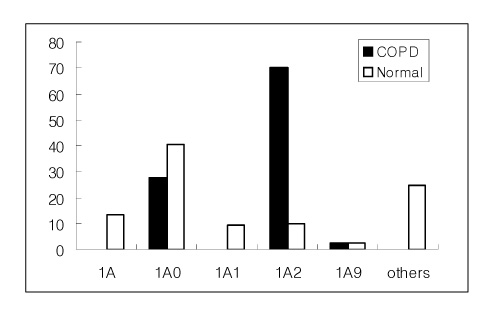
The specific frequencies of the 6A2 and 6A18 alleles of SP-A1 and the 1A2 allele of SP-A2 were much higher in the COPD group than control group (p<0.05). However, the frequencies of the 6A3 and 6A4 alleles of SP-A1 and the 1A0 allele of SP-A2 in the COPD group were significantly lower than the control group. In the COPD group, the frequencies of the +50 locus genotypes GG of SP-A1 and the +9 locus genotypes CC of SP-A2 were 85.0% and 60.6%, respectively, and 19.7% and 24.8% in the control group, respectively. The frequencies of the polymorphic genotypes or alleles showed a statistically significant difference between the COPD group and the control group (P<0.05).
CONCLUSION
A genetic polymorphism in SP-A is associated with the development of COPD in the Korean population.
Keyword
MeSH Terms
Figure
Reference
-
1. ATS Statement. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease: definitions, epidemiology, pathophysiology, diagnosis, and staging. Am J Respir Crit Care Med. 1995. 152:S77–S121.2. Bascom R. Differential susceptability to tobacco smoke: possible mechanisms. Pharmacogenetics. 1991. 1:102–106.3. Grese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999. 13:1455–1476.4. Behera D, Balamugesh T, Venkateswarlu D, Gupta A, Majumdar S. Serum surfactant protein-A levels in chronic bronchitis and its relation to smoking. Indian J Chest Dis Allied Sci. 2005. 47:13–17.5. Wirtz H, Habscheid W, Ertl G, Schmidt M, Kochsiek K. Exogenous surfactant application in respiratory failure due to chronic obstructive pulmonary disease. Respiration. 1995. 62:157–159.6. Otto-Verberne CJ, Ten Have-Opbroek AA, Franken C, Hermans J, Dijkman JH. Protective effect of pulmonary surfactant on elastase-induced emphysema in mice. Eur Respir J. 1992. 5:1223–1230.7. Lusuardi M, Capelli A, Carli S, Tacconi MT, Salmona M, Donner CF. Role of surfactant in chronic obstructive pulmonary disease: therapeutic implications. Respiration. 1992. 59:Suppl. 28–32.8. Fujishima T, Takahashi H, Abe S. Cytokines and surfactant as a factor of onset and progression of COPD. Nippon Rinsho. 1999. 57:1976–1981.9. Xie JG, Xu YJ, Zhang ZX, Ni W, Chen SX. Surfactant protein A gene polymorphisms in chronic obstructive pulmonary disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005. 22:91–93.10. Seifart C, Plagens A, Brodje D, Muller B, von Wichert P, Floros J. Surfactant protein B intron 4 variation in German patients with COPD and acute respiratory failure. Dis Markers. 2002. 18:129–136.11. Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, et al. Surfactant protein gene A, B, and D marker alleles in chronic obstructive pulmonary disease of a Mexican population. Eur Respir J. 2001. 18:482–490.12. Guo X, Lin HM, Lin Z, Montano M, Sansores R, Wang G, et al. Polymorphisms of surfactant protein gene A, B, D, and of SP-B-linked microsatellite markers in COPD of a Mexican population. Chest. 2000. 117(5):Suppl 1. 249S–250S.13. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: NHLBI/WHO workshop report. No. 2701. Bethesda, National Heart, Lung and Blood Institute. 2001. NIH Publication;1–100.14. DiAngelo S, Lin Z, Wang G, Philips S, Ramet M, Luo J, et al. Novel, Non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Dis Markers. 1999. 15:269–281.15. Kim DS, Kim YS, Jung KS, Chang JH, Lim CM, Lee JH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med. 2005. 172:842–847.16. Bascom R. Differential susceptibility to tobacco smoke: possible mechanism. Pharmacogenetics. 1991. 1:102–106.17. Wirtz H, Habscheid W, Ertl G, Schmidt M, Kochsiek K. Exogenous surfactant application in respiratory failure due to chronic obstructive pulmonary disease. Respiration. 1995. 62:157–159.18. Lee KS, Kim YH, Suk JS, Ko JH, OH MH, Baw CW. Allele distribution and frequency of human surfactant protein-AI in Korean neonates. J Korean Pediatr Soc. 2002. 45:1797–1502.19. Kim NC, Yoon HC, Suk JS, Ko JH, Yoo OJ, Lee IK, Oh MH, Bae CW. Allele distribution and freguency of human surfactant protein-Azin Korean neonates. J Korean Pediatr Soc. 2003. 46:340–344.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Surfactant Protein-A(SP-A) Gene Locus Associated with Mycoplasma pneumoniae Pneumonia in Korean Children
- Association between the Human Surfactant Protein-A(SP-A) Gene Locus and Respiratory Distress Syndrome in Korean Neonates
- Effect of Dexamethasone on Gene Expression of Surfactant Protein B and Surfactant Protein C
- Up-regulation of Surfactant Protein-A in Chronic Sialadenitis
- The comparative assessment of nuclear run on of hydrophilic and hydrophobic surfactant protein by administration of steroid