J Korean Endocr Soc.
2007 Apr;22(2):118-124. 10.3803/jkes.2007.22.2.118.
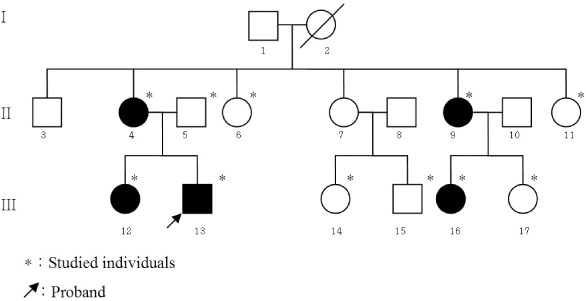
A Novel Mutation of the Vasopressin-Neurophysin II Gene in a Familial Neurohypophyseal Diabetes Insipidus
- Affiliations
-
- 1Department of Internal Medicine, Hallym University School of Medicine, Korea.
- 2Department of Internal Medicine1, KyungHee University College of Medicine, Korea.
- KMID: 1523092
- DOI: http://doi.org/10.3803/jkes.2007.22.2.118
Abstract
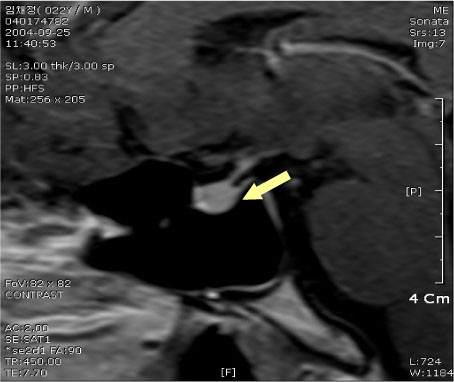
- Autosomal dominant familial neurohypophyseal diabetes insipidus (adFNDI) is a rare form of central diabetes insipidus (DI), and this malady is clinically characterized by polydipsia and polyuria, and it is caused by mutation in the vasopressin-neurophysin II. We identified a Korean family that suffered with adFNDI and we found a novel mutation in the NP II molecule. The index subject's DI symptoms dated to childhood, and his familial history was consistent with autosomal transmission. The diagnosis of central DI was done by performing a water deprivation test and a vasopressin challenge test. For molecular analysis, the genomic DNA was extracted and the AVP-NP II gene was amplified by polymerase chain reaction from four clinically-affected members and seven clinically-nonaffected members. Genetic analysis of AVP-NP II revealed new a heterozygous missense mutation in exon 2 of the AVP-NP II gene (+1692C > A) and this amino acid substitution (Cys105Stop) was predicted to have occurred in four clinically-affected subjects. In summary, in the present study we have described a novel mutation of the AVP-NPII gene in a Korean family suffering with adFNDI.
MeSH Terms
Figure
Reference
-
1. Jeon HJ, Park SH, Kwon SH, Lee SH, Park KS, Kim SY, Lee HK. Clinical course of idiopathic central diabetes insipidus in adults. J Kor Soc Endocrinol. 2001. 16:190–198.2. Kim B-W. Idiopathic central diabetes insipidus in adults. J Kor Soc Endocrinol. 2001. 16:185–189.3. Ito M, Yu RN, Jameson JL. Mutant vasopressin precursors that cause autosomal dominant 0neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem. 1999. 274:9029–9037.4. Olias G, Richter D, Schmale H. Heterologous expression of human vasopressin-neurophysin precursors in a pituitary cell line: defective transport of a mutant protein from patients with familial diabetes insipidus. DNA Cell Biol. 1996. 15:929–935.5. Nijenhuis M, Zalm R, Burbach JP. A diabetes insipidus vasopressin prohormone altered outside the central core of neurophysin accumulates in the endoplasmic reticulum. Mol Cell Endocrinol. 2000. 167:55–67.6. Siggaard C, Rittig S, Corydon TJ, Andreasen PH, Jensen TG, Andresen BS, Robertson GL, Gregersen N, Bolund L, Pedersen EB. Clinical and molecular evidence of abnormal processing and trafficking of the vasopressin preprohormone in a large kindred with familial neurohypophyseal diabetes insipidus due to a signal peptide mutation. J Clin Endocrinol Metab. 1999. 84:2933–2941.7. Beuret N, Rutishauser J, Bider MD, Spiess M. Mechanism of endoplasmic reticulum retention of mutant vasopressin precursor caused by a signal peptide truncation associated with diabetes insipidus. J Biol Chem. 1999. 274:18965–18972.8. Baglioni S, Corona G, Maggi M, Serio M, Peri A. Identification of a novel mutation in the arginine vasopressin-neurophysin II gene affecting the sixth intrachain disulfide bridge of the neurophysin II moiety. Eur J Endocrinol. 2004. 151:605–611.9. Tae HJ, Baek KH, Shim SM, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK. A novel splice site mutation of the arginine vasopressin-neurophysin II gene identified in a kindred with autosomal dominant familial neurohypophyseal diabetes insipidus. Mol Genet Metab. 2005. 86:307–313.10. Wahlstrom JT, Fowler MJ, Nicholson WE, Kovacs WJ. A novel mutation in the preprovasopressin gene identified in a kindred with autosomal dominant neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab. 2006. 89:1963–1968.11. Baylis PH, Cheetham T. Diabetes insipidus. Arch Dis Child. 1998. 79:84–89.12. Davies JH, Penney M, Abbes AP, Engel H, Gregory JW. Clinical Features, Diagnosis and Molecular Studies of Familial Central Diabetes Insipidus. Horm Res. 2005. 64:231–237.13. Si-Hoe SL, De Bree FM, Nijenhuis M, Davies JE, Howell LM, Tinley H, Waller SJ, Zeng Q, Zalm R, Sonnemans M, Van Leeuwen FW, Burbach JP, Murphy D. Endoplasmic reticulum derangement in hypothalamic neurons of rats expressing a familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J. 2000. 14:1680–1684.14. Davies J, Murphy D. Autophagy in hypothalamic neurones of rats expressing a familial neurohypophyseal diabetes insipidus transgene. J Neuroendocrinol. 2002. 14:629–637.15. Russell TA, Ito M, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL. A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest. 2003. 112:1697–1706.16. Siggaard C, Christensen JH, Corydon TJ, Rittig S, Robertson GL, Gregersen N, Bolund L, Pedersen EB. Expression of three different mutations in the arginine vasopressin gene suggests genotype-phenotype correlation in familial neurohypophyseal diabetes insipidus kindreds. Clin Endocrinol. 2005. 63:207–216.17. Fujisawa I, Nishimura K, Asato R, Togashi K, Itoh K, Noma S, Kawamura Y, Sago T, Minami S, Nakano Y, Itoh H, Torizuka K. Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr. 1987. 11:221–225.18. Miyamoto S, Sasaki N, Tanabe Y. Magnetic resonance imaging in familial central diabetes insipidus. Neuroradiology. 1991. 33:272–273.19. Maghnie M, Villa A, Arico M, Larizza D, Pezzotta S, Beluffi G, Genovese E, Severi F. Correlation between magnetic resonance imaging of posterior pituitary and neurohypophyseal function in children with diabetes insipidus. J Clin Endocrinol Metab. 1992. 74:795–800.20. Gagliardi PC, Bernasconi S, Repaske DR. Autosomal dominant neurohypophyseal diabetes insipidus associated with a missense mutation encoding Gly23 → Val in neurophysin II. J Clin Endocrinol Metab. 1997. 82:3643–3646.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autosomal dominant familial neurohypophyseal diabetes insipidus caused by a mutation in the arginine-vasopressin II gene in four generations of a Korean family
- A Familial Case of Nephrogenic Diabetes Insipidus Associated with a Mutation of the AVPR2 Gene
- A Case of Langerhan's Symbol 146/f "Times New Roman" Cell Histiocytosis with Diabetes Insipidus
- A Case of Idiopathic Central Diabetes Insipidus together with Primary Empty Sella and Combined Pituitary Hormone Deficiency
- Congenital Nephrogenic Diabetes Insipidus Developing in the Mother and the Son