Cancer Res Treat.
2007 Sep;39(3):134-137.
A Locally Advanced Breast Cancer with Difficult Differential Diagnosis of Carcinosarcoma and Atypical Medullary Carcinoma, which had Poor Response to Adriamycin- and Taxane-based Neoadjuvant Chemotherapy: A Case Report
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. Jeunghc1123@yuhs.ac
- 2Cancer Metastasis Research Center, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Hongik Hospital, Seoul, Korea.
Abstract
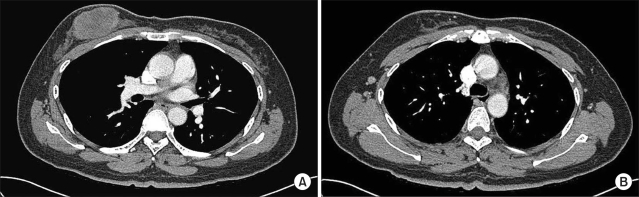
- Atypical medullary carcinomas and carcinosarcoma have unique histopathological features. Here we present a case with a breast malignancy that had pathological characteristics of both. A 54-year old patient with a malignant breast mass received 6 cycles of adriamycin-based chemotherapy, followed by 3 cycles of paclitaxel monotherapy, and had a poor clinical response to treatment. A modified radical mastectomy was performed. The pathological diagnosis was complicated by an inability to distinguish between atypical medullary carcinoma and carcinosarcoma. The findings included a tumor that was well-circumscribed, high grade and a syncytial growth pattern as well as biphasic sarcomatous and carcinomatous characteristics. In conclusion, atypical medullary carcinoma and carcinosarcoma of the breast have entirely different prognoses and should be managed differently. Both should be treated by surgical resection, and additional therapy should be considered based on the cancer with the poorer prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Reinfuss M, Stelmach A, Mitus J, Rys J, Duda K. Typical medullary carcinoma of the breast: a clinical and pathological analysis of 52 cases. J Surg Oncol. 1995; 60:89–94. PMID: 7564387.
Article2. Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V. Medullary carcinoma of the breast: a clinicopatholigic study with 10 year follow-up. Cancer. 1977; 40:1365–1385. PMID: 907958.3. Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005; 93:1046–1052. PMID: 16175185.
Article4. Vu-Nishino H, Tavassoli FA, Ahrens WA, Haffty BG. Clinicopathologic features and long-term outcome of patients with medullary breast carcinoma managed with breast-conserving therapy (BCT). Int J Radiat Oncol Biol Phys. 2005; 62:1040–1047. PMID: 15990007.
Article5. Gutman H, Pollock RE, Janjan NA, Johnston DA. Biologic distinctions and therapeutic implications of sarcomatoid metaplasia of epithelial carcinoma of the breast. J Am Coll Surg. 1995; 180:193–199. PMID: 7850054.6. Kim SW, Kang HJ, Youn YK, Oh SK, Choe KJ, Noh DY. The clinicopathologic characteristics of metaplastic carcinomas of the breast. J Korean Surg Soc. 2001; 60:251–255.7. Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, et al. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006; 17:605–613. PMID: 16469754.
Article8. Wargotz ES, Norris HJ. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer. 1989; 64:1490–1499. PMID: 2776108.
Article9. Rayson D, Adjei AA, Suman VJ, Wold LE, Ingle JN. Metaplastic breast cancer: Prognosis and response to systemic therapy. Ann Oncol. 1999; 10:413–419. PMID: 10370783.
Article10. Gaffey MJ, Mills SE, Frierson HF, Zarbo RJ, Boyd JC, Simpson JF, et al. Medullary carcinoma of the breast: Interobserver variability in histopathologic diagnosis. Mod Pathol. 1995; 8:31–38. PMID: 7731939.11. Jensen ML, Kiaer H, Andersen J, Jensen V, Melsen F. Prognostic comparison of three classifications for medullary carcinomas of the breast. Histopathology. 1997; 30:523–532. PMID: 9205856.
Article12. Domagala W, Wozniak L, Lasota J, Weber K, Osbron M. Vimentin is preferentially expressed in high-grade ductal and medullary, but not in lobular breast carcinomas. Am J Pathol. 1990; 137:1059–1064. PMID: 2173410.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- Short Term Effect of Neoadjuvant Therapy with Docetaxel and Adriamycin in Advanced Breast Cancer
- Predictive Factors for Non-Response to Neoadjuvant Chemotherapy for Breast Cancer
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin
- Transcatheter Arterial Embolization for the Control of Neoplastic Hemorrhage in Locally Advanced Breast Cancer: A Case Report