Yonsei Med J.
2013 Mar;54(2):321-329. 10.3349/ymj.2013.54.2.321.
Intrathecal Lamotrigine Attenuates Mechanical Allodynia and Suppresses Microglial and Astrocytic Activation in a Rat Model of Spinal Nerve Ligation
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jongyeon_park@amc.seoul.kr
- KMID: 1503892
- DOI: http://doi.org/10.3349/ymj.2013.54.2.321
Abstract
- PURPOSE
Lamotrigine, a novel anticonvulsant, is a sodium channel blocker that is efficacious in certain forms of neuropathic pain. Recently, microglial and astrocytic activation has been implicated in the development of nerve injury-induced neuropathic pain. We have assessed the effects of continuous intrathecal administration of lamotrigine on the development of neuropathic pain and glial activation induced by L5/6 spinal-nerve ligation in rats.
MATERIALS AND METHODS
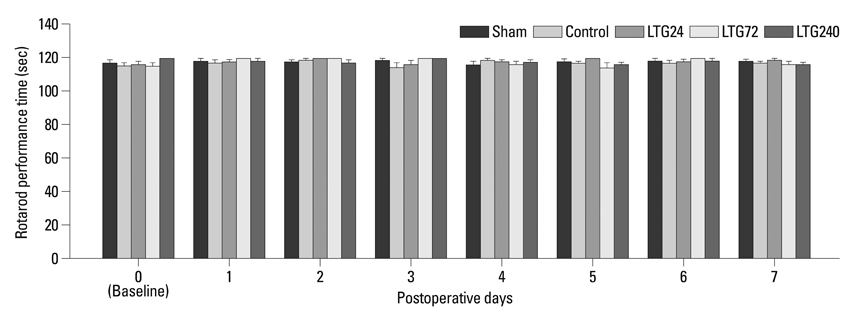
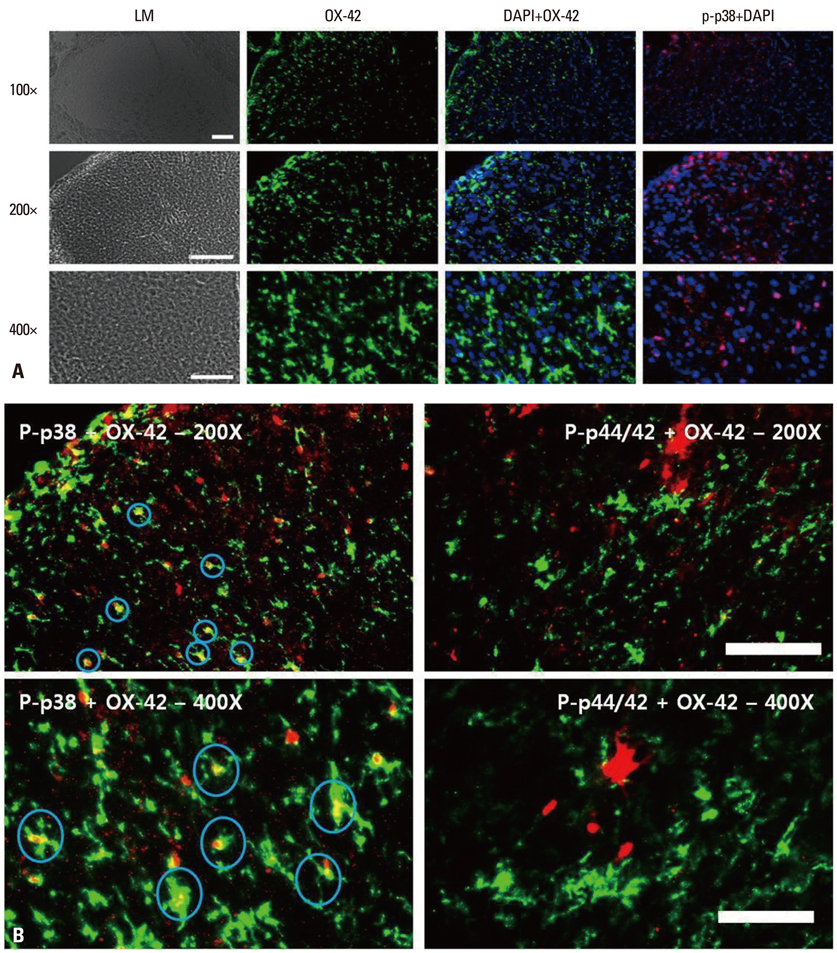
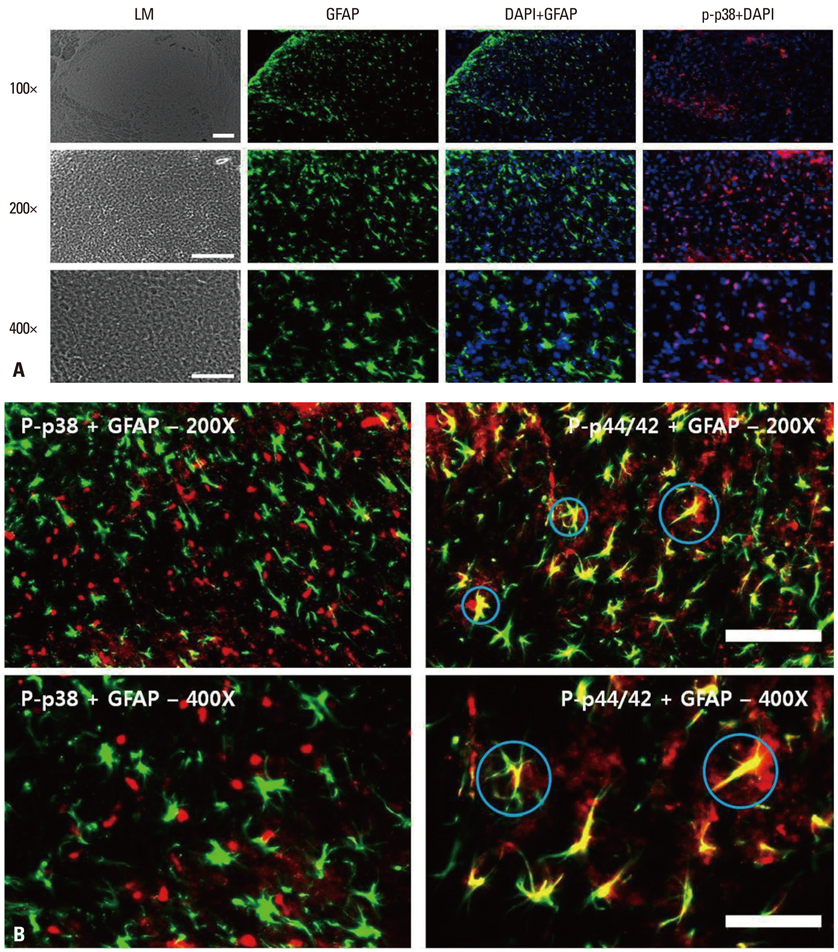
Following left L5/6 spinal nerve ligation (SNL), Sprague-Dawley male rats were intrathecally administered lamotrigine (24, 72, or 240 microg/day) or saline continuously for 7 days. Mechanical allodynia of the left hind paw to von Frey filament stimuli was determined before surgery (baseline) and once daily for 7 days postoperatively. On day 7, spinal activation of microglia and astrocytes was evaluated immunohistochemically, using antibodies to the microglial marker OX-42 and the astrocyte marker glial fibrillary acidic protein (GFAP).
RESULTS
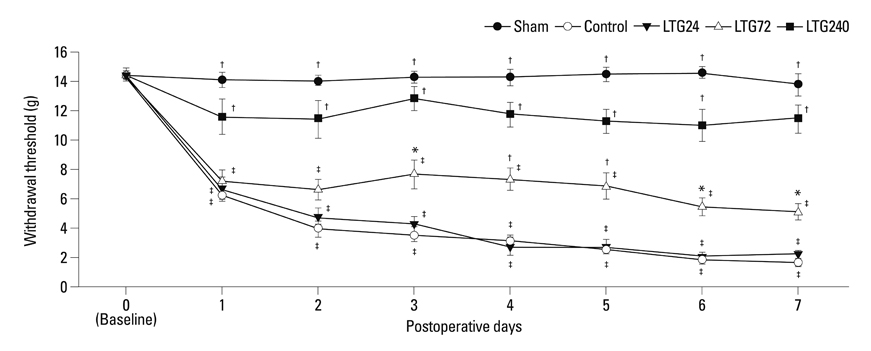
Spinal-nerve ligation induced mechanical allodynia in saline-treated rats, with OX-42 and GFAP immunoreactivity being significantly increased on the ipsilateral side of the spinal cord. Continuously administered intrathecal lamotrigine (240 microg/day) prevented the development of mechanical allodynia, and lower dose of lamotrigine (72 microg/day) ameliorated allodynia. Intrathecal lamotrigine (72 and 240 microg/day) inhibited nerve ligation-induced microglial and astrocytic activation, as evidenced by reduced numbers of cells positive for OX-42 and GFAP.
CONCLUSION
Continuously administered intrathecal lamotrigine blocked the development of mechanical allodynia induced by SNL with suppression of microglial and astrocytic activation. Continuous intrathecal administration of lamotrigine may be a promising therapeutic intervention to prevent neuropathy.
Keyword
MeSH Terms
-
Animals
Astrocytes/drug effects/*physiology
Disease Models, Animal
Hyperalgesia/*drug therapy
Infusions, Spinal
Ligation
Male
Microglia/drug effects/*physiology
Neuralgia/drug therapy
Rats
Rats, Sprague-Dawley
Spinal Nerves/*injuries
Triazines/administration & dosage/*therapeutic use
Voltage-Gated Sodium Channel Blockers/administration & dosage/*therapeutic use
Triazines
Voltage-Gated Sodium Channel Blockers
Figure
Reference
-
1. Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006. 26:3551–3560.2. Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999. 157:289–304.
Article3. Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005. 6:521–532.
Article4. Lees G, Leach MJ. Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res. 1993. 612:190–199.
Article5. McCleane GJ. Lamotrigine in the management of neuropathic pain: a review of the literature. Clin J Pain. 2000. 16:321–326.
Article6. Datta S, Waghray T, Torres M, Glusman S. Amiodarone decreases heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Anesth Analg. 2004. 98:178–184.
Article7. Ma W, Du W, Eisenach JC. Intrathecal lidocaine reverses tactile allodynia caused by nerve injuries and potentiates the antiallodynic effect of the COX inhibitor ketorolac. Anesthesiology. 2003. 98:203–208.
Article8. Klamt JG. Effects of intrathecally administered lamotrigine, a glutamate release inhibitor, on short- and long-term models of hyperalgesia in rats. Anesthesiology. 1998. 88:487–494.
Article9. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.
Article10. Størkson RV, Kjørsvik A, Tjølsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996. 65:167–172.
Article11. Lee TH, Wang CJ, Wu PC, Buerkle H, Lin SH, Yang LC. The thermal and mechanical anti-hyperalgesic effects of pre- versus post-intrathecal treatment with lamotrigine in a rat model of inflammatory pain. Life Sci. 2002. 70:3039–3047.
Article12. Song JG, Jun IG, Kwon MY, Park JY. The mechanical antiallodynic effect of intrathecal lamotrigine in rats with spinal nerve ligation. Korean J Pain. 2005. 18:118–124.
Article13. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.
Article14. Teoh H, Fowler LJ, Bowery NG. Effect of lamotrigine on the electrically-evoked release of endogenous amino acids from slices of dorsal horn of the rat spinal cord. Neuropharmacology. 1995. 34:1273–1278.
Article15. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999. 353:1959–1964.
Article16. Erichsen HK, Hao JX, Xu XJ, Blackburn-Munro G. A comparison of the antinociceptive effects of voltage-activated Na+ channel blockers in two rat models of neuropathic pain. Eur J Pharmacol. 2003. 458:275–282.
Article17. Hilas O, Charneski L. Lamotrigine-induced Stevens-Johnson syndrome. Am J Health Syst Pharm. 2007. 64:273–275.
Article18. Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005. 114:149–159.
Article19. Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, et al. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem. 2006. 97:1337–1348.
Article20. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996. 19:312–318.
Article21. Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003. 104:655–664.
Article22. Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003. 23:4017–4022.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Mechanical Antiallodynic Effect of Intrathecal Lamotrigine in Rats with Spinal Nerve Ligation
- Intrathecal Lamotrigine Attenuates Antinociceptive Morphine Tolerance and Suppresses Spinal Glial Cell Activation in Morphine-Tolerant Rats
- The Mechanical Antiallodynic Effect of intrathecal Morphine and R-Phenylisopropyl-Adenosine in Rats with Spinal Nerve Ligation
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain
- The Mechanism of Antiallodynic Effect of Intrathecal Morphine in Neuropathic Pain Induced by Spinal Nerve Ligation