Ann Clin Microbiol.
2013 Sep;16(3):140-144. 10.5145/ACM.2013.16.3.140.
Evaluation of the Xpert Flu for the Detection of Influenza A Virus and Influenza A/H1N1/2009 Strain
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Sungkyunkwan University School of Medicine, Seoul, Korea. mrmicro@skku.edu
- 2Center for Clinical Medicine, Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- KMID: 1494331
- DOI: http://doi.org/10.5145/ACM.2013.16.3.140
Abstract
- BACKGROUND
Xpert Flu (Cepheid, USA) allows for fully automated real-time RT-PCR using a single-use disposable cartridge. The aim of this study was to evaluate Xpert Flu for the detection of influenza A virus and subtype A/H1N1/2009 pandemic virus.
METHODS
We conducted a prospective comparison study for Xpert Flu with the RealTime ready Influenza A/H1N1 Detection Set (Roche Diagnostics, Germany). Analytical specificities of the assays were determined by testing commonly encountered respiratory viral pathogens, including parainfluenza virus type 1/2/3, rhinovirus A, rhinovirus B, metapneumovirus, adenovirus, and coronavirus. The analytical sensitivities and workflow of both methods were also assessed.
RESULTS
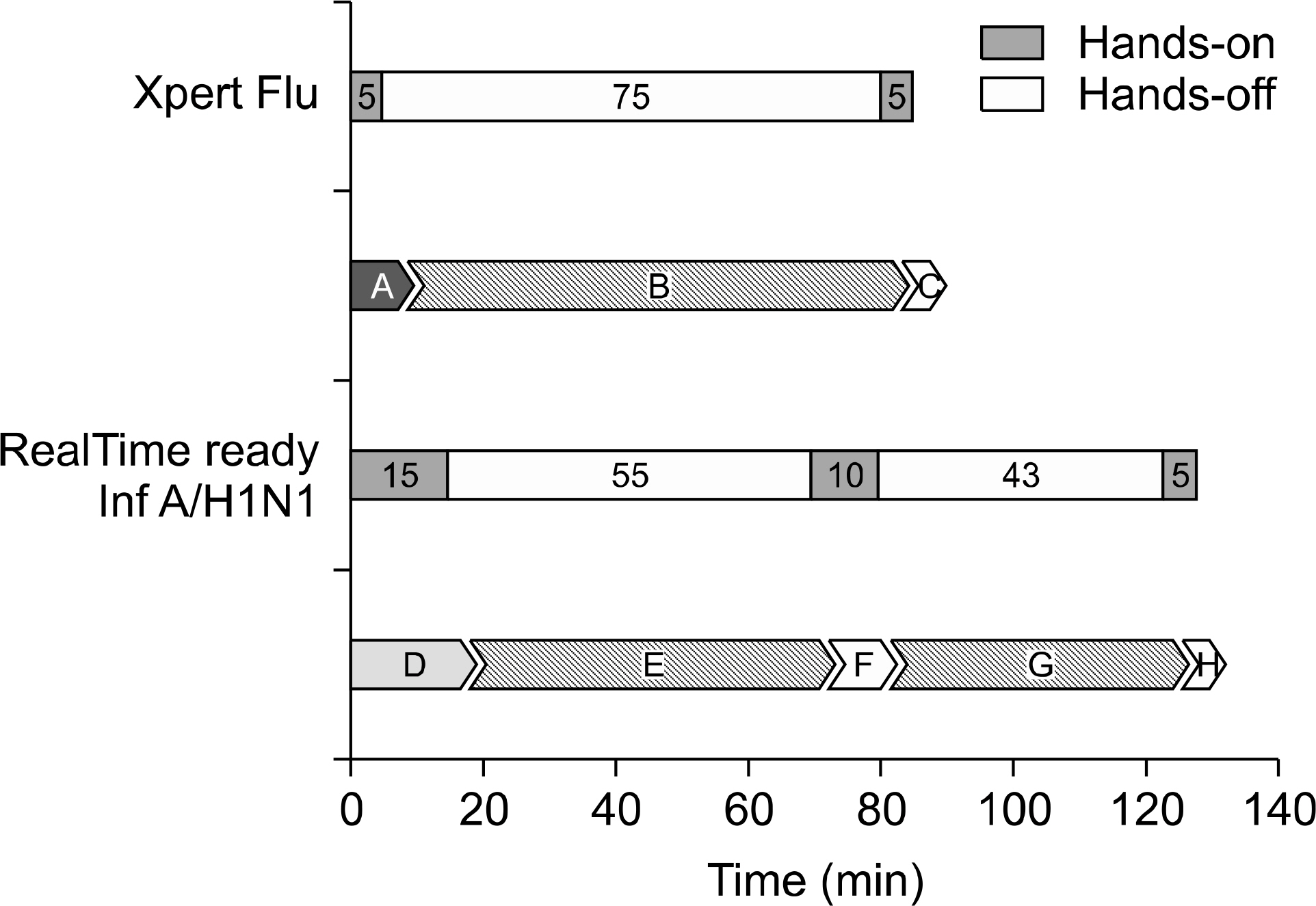
A total of 102 consecutive clinical specimens were tested by both methods. Total agreement between the two methods was estimated to be 99.0% (101/102): 11 A/H1N1/2009 and 3 seasonal influenza A by the RealTime ready Influenza A/H1N1 Detection Set; 10 and 3 by Xpert Flu. No cross-reactivity was observed between influenza A/H1N1/2009 and other respiratory viral pathogens in either method. The limits of detection of the RealTime ready Influenza A/H1N1 Detection Set and Xpert Flu were 500 TCID50/mL and 20 TCID50/mL, respectively. Xpert Flu required 85 minutes (10 minutes of hands-on time) for processing, while RealTime ready Influenza A/H1N1 Detection Set took 128 minutes (30 minutes of handson time).
CONCLUSION
The results of Xpert Flu were comparable to those of the RealTime ready Influenza A/H1N1 Detection Set. It is of note that the fully automated and closed system of Xpert Flu could be advantageous for reducing hands-on time and for preventing cross-contamination during the testing process.
MeSH Terms
Figure
Reference
-
1.Thompson WW., Shay DK., Weintraub E., Brammer L., Cox N., Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003. 289:179–86.
Article2.Muscatello DJ., Newall AT., Dwyer DE., Macintyre CR. Mortality attributable to seasonal and pandemic influenza, Australia, 2003 to 2009, using a novel time series smoothing approach. PLoS One. 2013. 8:e64734.
Article3.Yi K., Kang JO., Oh JW., Ham SY., Choi TY. Trends of viral respiratory pathogens detected in pediatric patients, 1996 through 2001. Korean J Clin Microbiol. 2002. 5:77–83.4.WHO on behalf of the Special Programme for Research and Training in Tropical Diseases. 2010. Use of Influenza Rapid Diagnostic Tests.http://whqlibdoc.who.int/publications/2010/9789241599283_eng.pdf/. [Online] (last visited on 6 August, 2013).5.Treanor JJ. Influenza Virus, Including Avian Influenza and Swine Influenza. Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. New York: Churchill Livingstone Inc;2010. p. 2265–88.6.Kwon A., Kim JS., Kim HS., Song W., Park JY., Cho HC, et al. Comparison of rapid antigen test and real-time reverse transcriptase PCR for diagnosing novel swine influenza A (H1N1). Korean J Clin Microbiol. 2010. 13:109–13.
Article7.Popowitch EB., Rogers E., Miller MB. Retrospective and prospective verification of the Cepheid Xpert influenza virus assay. J Clin Microbiol. 2011. 49:3368–9.
Article8.Salez N., Ninove L., Thirion L., Gazin C., Zandotti C., de Lamballerie X, et al. Evaluation of the Xpert Flu test and comparison with in-house real-time RT-PCR assays for detection of influenza virus from 2008 to 2011 in Marseille, France. Clin Microbiol Infect. 2012. 18:E81–3.
Article9.Li M., Brenwald N., Bonigal S., Chana K., Osman H., Oppenheim B. Rapid diagnosis of influenza: an evaluation of two commercially available RT-PCR assays. J Infect. 2012. 65:60–3.
Article10.Sambol AR., Iwen PC., Pieretti M., Basu S., Levi MH., Gilonske KD, et al. Validation of the Cepheid Xpert Flu A real time RT-PCR detection panel for emergency use authorization. J Clin Virol. 2010. 48:234–8.
Article11.Jenny SL., Hu Y., Overduin P., Meijer A. Evaluation of the Xpert Flu A Panel nucleic acid amplification-based point-of-care test for influenza A virus detection and pandemic H1 subtyping. J Clin Virol. 2010. 49:85–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influenza Associated Pneumonia
- The 2009 H1N1 Pandemic Influenza in Korea
- Novel swine-origin H1N1 influenza
- Clinical Characteristic of Respiratory Tract Infections in Children during Pandemic Influenza (H1N1 2009) in Korea
- Interpretation of Positive Result for Influenza A and Negative Result for Novel Influenza A/H1N1 in Reverse Transcriptase PCR for Novel Influenza A/H1N1