J Korean Surg Soc.
2009 Apr;76(4):236-245. 10.4174/jkss.2009.76.4.236.
Germline Genetic Alterations in Intraductal Papillary Neoplasms Associated with Extrapancreatic Tumors
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. sunkim@plaza.snu.ac.kr
- 2Labgenomics Clinical Research Institute, Seoul, Korea.
- KMID: 1465062
- DOI: http://doi.org/10.4174/jkss.2009.76.4.236
Abstract
-
PURPOSE: IPMN (Intraductal papillary mucinous neoplasm) is frequently reported in combination with a variety of extrapancreatic tumors. The IPMN in these patients might represent the phenotypes of genes associated with multiple tumor syndrome. The aim of this study was to confirm the presence of germline mutations in the p53, MLH1, MSH2, BRCA1/2, and E-cadherin genes known to be associated with gastrointestinal malignancies in hereditary tumor syndromes such as Li-Fraumeni syndrome, HNPCC, Hereditary Breast/Ovarian cancer, and Hereditary diffuse gastric cancer.
METHODS
14 patients with IPMN with extrapancreatic tumors (6 gastric cancers, 5 colorectal cancers, 1 gastric GIST, 2 hepatocellular carcinomas, 1 AoV cancer) who underwent resection were enrolled in this study. We performed PCR (Polymerase chain reaction) and direct sequencing analysis for the p53, MLH1, MSH2 and CDH-1 genes. Multiplex PCR, F-CSGE (fluorescent conformation sensitive gel electrophoresis) and direct sequencing was performed for BRCA1/2 genes.
RESULTS
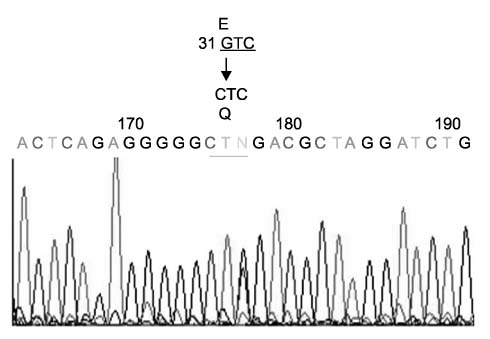
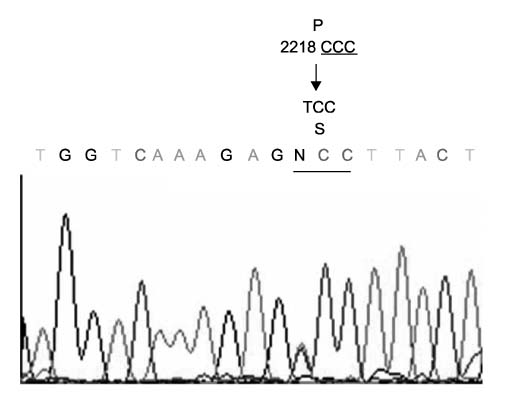
We identified two novel mutations in the p53 gene (exon 1, codon 31, GTC>CTC, Glu-->Gln) and the CDH-1 gene (exon 14, codon 2218, CCC>TCC, Pro-->Ser). For BRCA1, we identified 11 identical coding SNP (exon 11, codon 3232, AAG>AGG, Glu-->Gly) among 13 patients with a high allele frequency (46.1%) compared with the 30.1% reported in Korean breast cancer patients. For BRCA2, we identified a coding SNP with an allele frequency of 2.6% (exon 11, codon 2578, AAG>AGG, Met-->Val).
CONCLUSION
Germline alterations of the p53 and E-Cadherin genes in IPMN patients with extrapancreatic cancer suggest that IPMN could be a manifestation of multiple tumor syndrome.
Keyword
MeSH Terms
Figure
Reference
-
1. Jiang ZL, Satoh K, Moriizumi S, Shimosegawa T, Koizumi M, Toyota T. An analysis of the diseases associated with mucin-producing tumors of the pancreas. J Jpn Panc Soc. 1996. 11:289–292.2. Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999. 94:470–473.3. Yamaguchi K, Yokohata K, Noshiro H, Chijiiwa K, Tanaka M. Mucinous cystic neoplasm of the pancreas or intraductal papillary-mucinous tumour of the pancreas. Eur J Surg. 2000. 166:141–148.4. Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004. 28:241–246.5. Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005. 12:124–132.6. Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005. 11:5688–5690.7. Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006. 141:51–56.8. Eguchi H, Ishikawa O, Ohigashi H, Tomimaru Y, Sasaki Y, Yamada T, et al. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006. 139:749–754.9. Seo WT, Lee SJ, Chung JP, Park YN, Yoon DS, Song JW, et al. A case of intraductal papillary mucinous tumor of the pancreas accompanied by gastric cancer. Korean J Gastrointest Endosc. 2000. 21:877–881.10. Jang JY, Kim SW, Ahn YJ, Yoon YS, Lee KU, Lee YJ, et al. Analysis of clinical features and factors predictive of malignancy in intraductal papillary mucinous tumor of the pancreas: multi-center analysis in Korea. Korean J Hepatobiliary Pancreat Surg. 2003. 7:1–11.11. Spitzer E, Abbaszadegan MR, Schmidt F, Hauser A, Buwitt U, Lauter FR, et al. Detection of BRCA1 and BRCA2 mutations in breast cancer families by a comprehensive two-stage screening procedure. Int J Cancer. 2000. 85:474–481.12. Malkin D, Li FP, Strong LC, Fraumeni JF Jr, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990. 250:1233–1238.13. Bang YJ, Kang SH, Kim TY, Jung CW, Oh SM, Choe KJ, et al. The first documentation of Li-Fraumeni syndrome in Korea. J Korean Med Sci. 1995. 10:205–210.14. Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994. 425:357–367.15. Casey G, Yamanaka Y, Friess H, Kobrin MS, Lopez ME, Buchler M, et al. p53 mutations are common in pancreatic cancer and are absent in chronic pancreatitis. Cancer Lett. 1993. 69:151–160.16. Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004. 22:735–742.17. The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999. 91:1310–1316.18. Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002. 94:1358–1365.19. Berman DB, Costalas J, Schultz DC, Grana G, Daly M, Godwin AK. A common mutation in BRCA2 that predisposes to a variety of cancers is found in both Jewish Ashkenazi and non-Jewish individuals. Cancer Res. 1996. 56:3409–3414.20. Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003. 95:214–221.21. Figer A, Irmin L, Geva R, Flex D, Sulkes J, Sulkes A, et al. The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer. 2001. 84:478–481.22. Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002. 94:1365–1372.23. Seo JH, Cho DY, Ahn SH, Yoon KS, Kang CS, Cho HM, et al. BRCA1 and BRCA2 germline mutations in Korean patients with sporadic breast cancer. Hum Mutat. 2004. 24:350.24. Sigurdson AJ, Hauptmann M, Chatterjee N, Alexander BH, Doody MM, Rutter JL, et al. Kin-cohort estimates for familial breast cancer risk in relation to variants in DNA base excision repair, BRCA1 interacting and growth factor genes. BMC Cancer. 2004. 4:9.25. Caldas C, Carneiro F, Lynch HT, Yokota J, Wiesner GL, Powell SM, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet. 1999. 36:873–880.26. Berx G, Staes K, van Hengel J, Molemans F, Bussemakers MJ, van Bokhoven A, et al. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1). Genomics. 1995. 26:281–289.27. Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, Grehan N, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004. 41:508–517.28. Yoon KA, Ku JL, Yang HK, Kim WH, Park SY, Park JG. Germline mutations of E-cadherin gene in Korean familial gastric cancer patients. J Hum Genet. 1999. 44:177–180.29. Li LC, Chui RM, Sasaki M, Nakajima K, Perinchery G, Au HC, et al. A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities. Cancer Res. 2000. 60:873–876.30. Park WS, Cho YG, Park JY, Kim CJ, Lee JH, Kim HS, et al. A single nucleotide polymorphism in the E-cadherin gene promoter-160 is not associated with risk of Korean gastric cancer. J Korean Med Sci. 2003. 18:501–504.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extrapancreatic Tumors in Intraductal Papillary Mucinous Neoplasm of the Pancreas
- Meaning of Incidence of Extrapancreatic Neoplasms in Patients with Intraductal Papillary Mucinous Neoplasms of Pancreas
- Comparison of Mucinous Cystic Tumor and Intraductal Papillary Mucinous Tumor
- How Can We Interpret the High Prevalence of Extrapancreatic Neoplasms in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas?
- Cystic Neoplasms and Intraductal Papillary Mucinous Neoplasms of the Pancreas